Understanding the Bair Hugger Medical Device
What is a bear hugger in medical terms refers to the Bair Hugger system, a forced-air warming (FAW) device used in hospitals to prevent hypothermia during surgery. The system includes a warming unit that blows heated, filtered air through disposable blankets placed on patients.
Quick Facts:
- Primary Function: Prevents perioperative hypothermia by maintaining a patient’s normal body temperature.
- Components: A reusable warming unit, a flexible hose, and single-use disposable blankets.
- Usage: Standard equipment in over 80% of U.S. hospitals, used on more than 300 million patients since its invention in 1987.
- Manufacturer: 3M Company.
While the Bair Hugger is a staple in operating rooms, it is now at the center of thousands of lawsuits. These lawsuits allege the device increases infection risk during surgeries, especially joint replacements.
Maintaining a normal body temperature during surgery is crucial for preventing complications like increased blood loss and higher infection rates. However, some patients who developed severe infections after hip or knee surgery claim the Bair Hugger system was a contributing factor.
What is the Bair Hugger System and How Does It Work?
When you hear “what is a bear hugger in medical terms,” you’re learning about a convective warming technology that uses forced-air warming (FAW) to keep patients safe during surgery. It actively fights perioperative hypothermia, a dangerous drop in body temperature that can occur during surgical procedures.
The Core Function: Preventing Perioperative Hypothermia
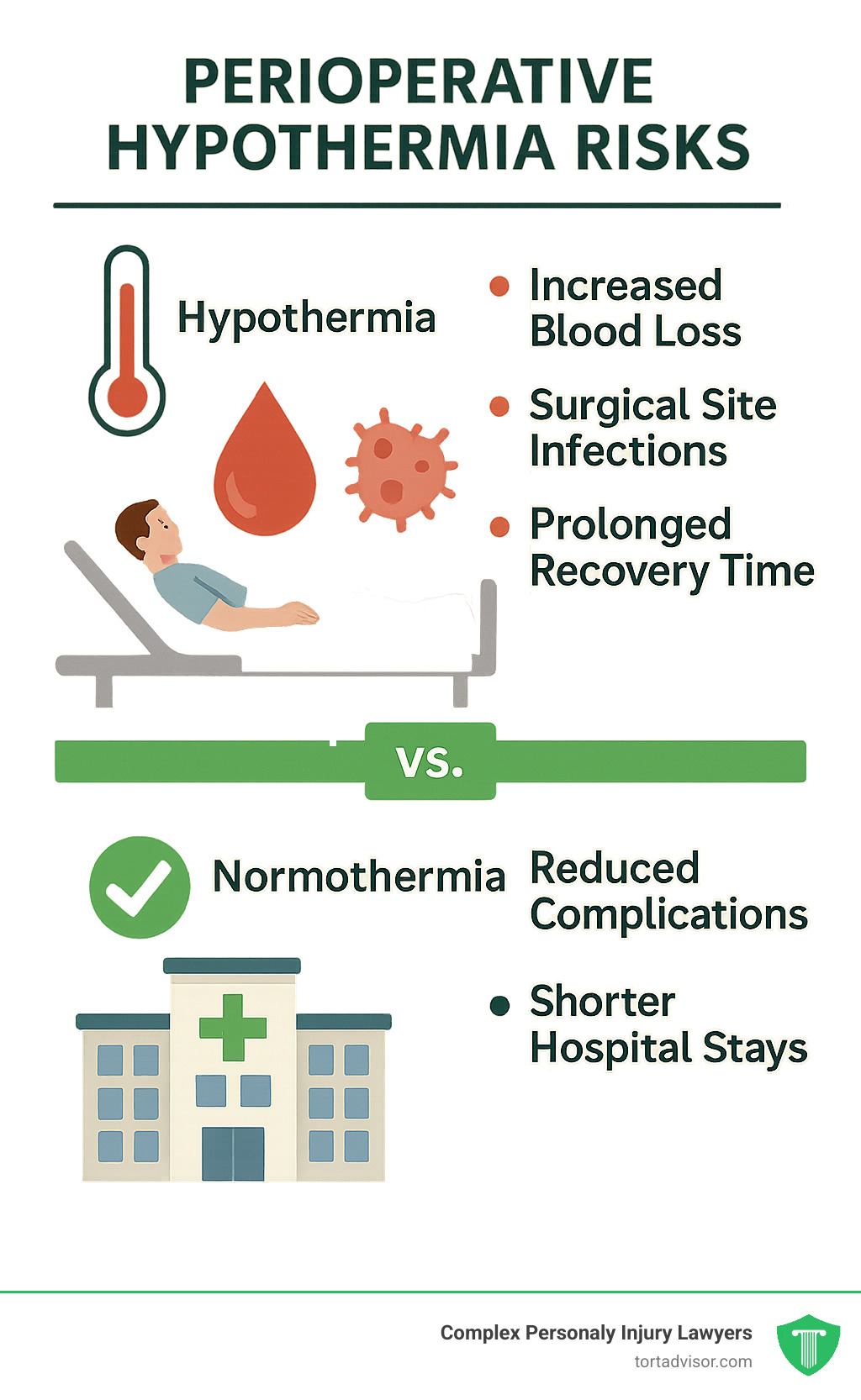
Perioperative hypothermia is a serious medical condition where a patient’s core body temperature drops below normal during surgery. Anesthesia interferes with the body’s natural temperature regulation, and the cool operating room environment exacerbates the issue.
The consequences of hypothermia are significant, including:
- Increased blood loss, potentially requiring transfusions.
- A higher surgical site infection risk.
- Prolonged recovery and longer hospital stays.
Maintaining normothermia (a normal body temperature) is essential for preventing these complications, which is the primary purpose of the Bair Hugger system.
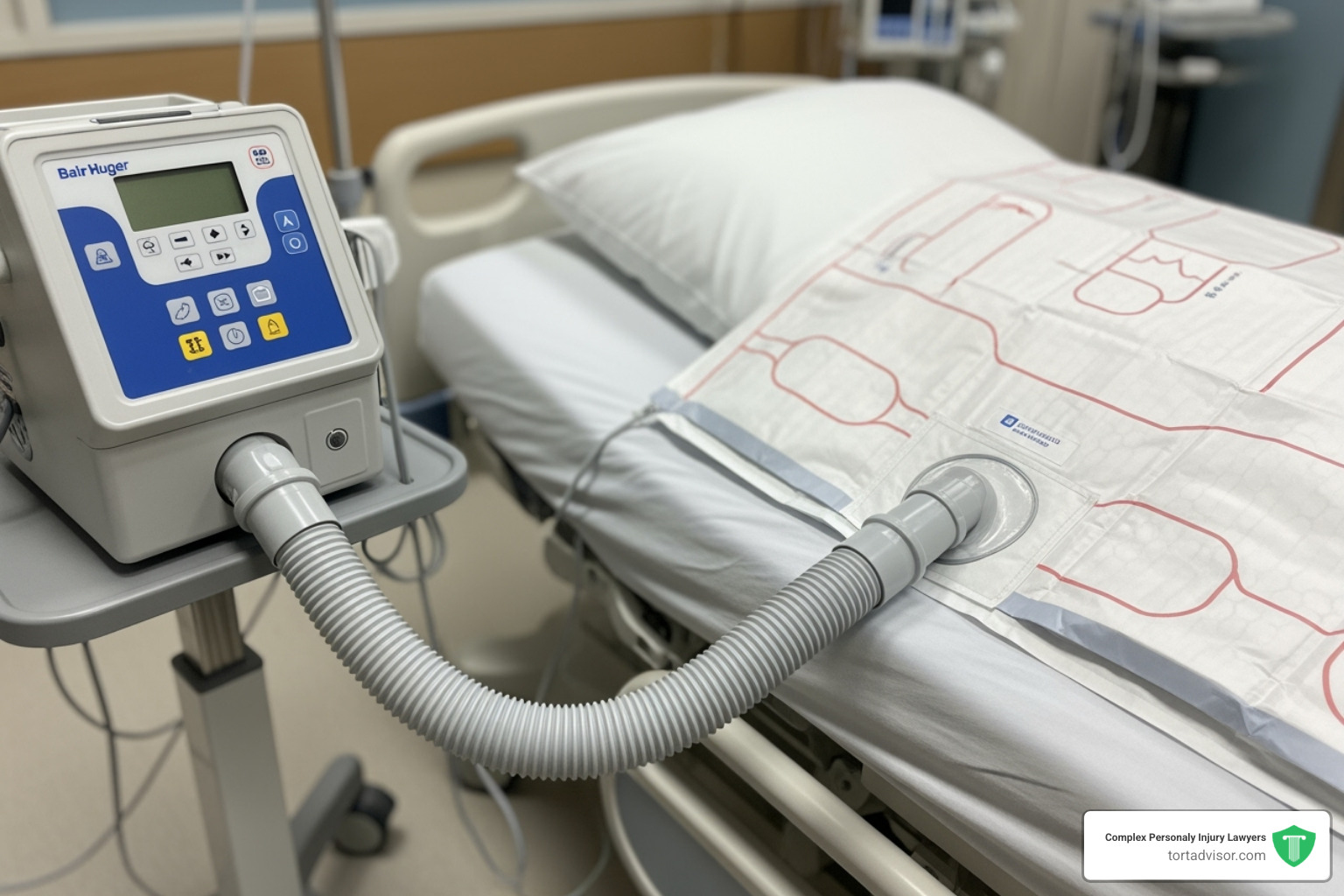
Components and Mechanism of Action
The Bair Hugger’s forced-air warming (FAW) technology uses three key components:
- Reusable warming unit: This unit draws in room air, cleans it with a HEPA filter, and heats it to a precise temperature.
- Flexible hose: It connects the warming unit to the blanket, delivering the heated air.
- Single-use disposable blankets: These blankets have thousands of tiny micro-perforations on the underside.
The warm, filtered air flows through the hose into the blanket and is gently released through the micro-perforations, creating a layer of warm air around the patient. This heat transfer process, known as convective warming, effectively maintains the patient’s core temperature throughout the surgical procedure.
The History and Widespread Adoption of Bair Hugger
The Bair Hugger system’s journey from a single inventor’s idea to an industry standard highlights its impact on modern surgical care.
From Invention to Industry Standard
In 1987, anesthesiologist Dr. Scott Augustine invented the Bair Hugger to solve the common problem of patients becoming hypothermic during surgery. He founded Augustine Medical (later Arizant Inc.) to market his invention. The medical community quickly adopted the device for its effectiveness and ease of use.
Today, the Bair Hugger’s success is evident:
- More than 80% of U.S. hospitals use the system.
- Over 300 million patients worldwide have been warmed with Bair Hugger technology.
In 2010, manufacturing giant 3M Company acquired Arizant Inc., incorporating the Bair Hugger into its extensive medical technology portfolio. The story of 3M’s acquisition of the Bair Hugger manufacturer marked a major milestone for the device.
FDA Clearance and Regulatory Oversight
The Bair Hugger received its initial FDA clearance in 1987 through the 510(k) clearance process. This pathway allows devices to be marketed if they are “substantially equivalent” to a legally marketed device, expediting patient access to beneficial innovations. The original 1987 FDA clearance document is publicly available.
In 2018, there was a recall of 165,000 Bair Hugger warming blankets. This recall was not related to infection concerns but addressed a design defect that prevented some blankets from inflating properly, which could reduce their warming effectiveness. This issue is separate from the infection risk allegations that have led to widespread litigation.
What is a Bear Hugger in Medical Terms? The Benefits vs. The Risks
Understanding what is a bear hugger in medical terms means looking at a device at the center of a medical debate, with extensive research supporting its benefits weighed against growing concerns about potential risks that have led to thousands of lawsuits.
The Scientific Case for Patient Warming
The medical evidence supporting patient warming during surgery is overwhelming. Maintaining normal body temperature provides significant benefits, including:
- Reduced Infections: Research on normothermia reducing surgical wound infections shows that keeping patients warm can dramatically lower the rate of surgical site infections.
- Less Blood Loss: Warm patients experience more normal blood clotting, reducing the need for blood transfusions.
- Faster Recovery: Patients tend to recover more quickly and have shorter hospital stays.
3M, the manufacturer, cites over 170 studies supporting the Bair Hugger’s safety and effectiveness.
The Controversy: Allegations of Increased Infection Risk
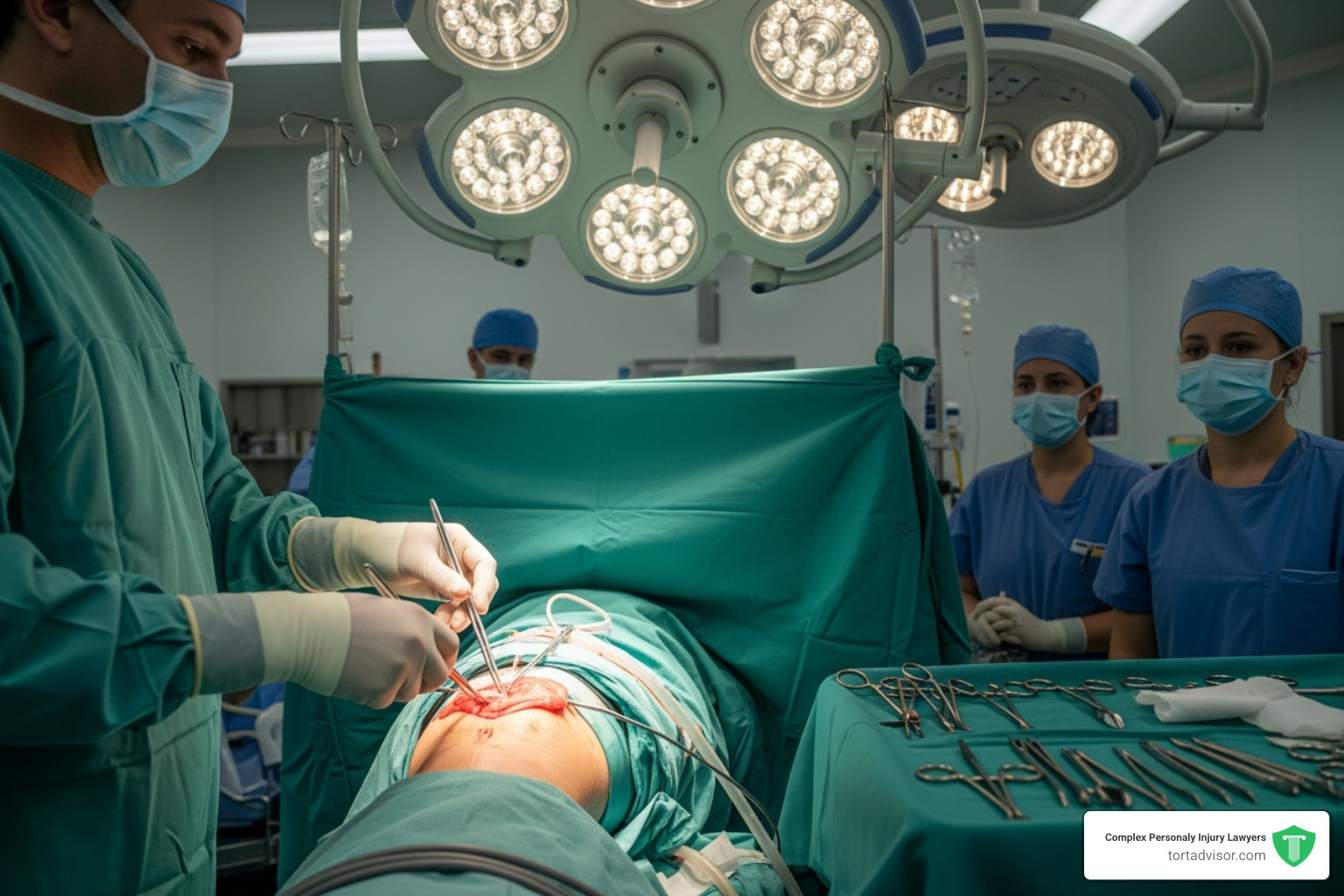
Despite the benefits, thousands of patients allege the Bair Hugger caused their surgical infections, particularly after hip and knee replacements. The concern focuses on the device’s forced-air warming system. Critics argue that the device’s air currents can disrupt the sterile operating room environment by picking up bacteria from the floor and circulating it into the surgical site.
Modern operating rooms use laminar flow systems to push clean air down and away from the patient. The fear is that the Bair Hugger’s upward airflow could interfere with this protective system. This is especially dangerous in joint replacement surgeries, where even a small amount of bacteria like MRSA can cause devastating deep joint infections, sometimes requiring additional surgeries or even amputation.
What is a bear hugger in medical terms regarding legal claims?
Thousands of patients have filed product liability lawsuits against 3M, claiming the Bair Hugger led to their post-surgical infections. These cases generally argue that 3M failed to warn doctors and patients about a known design defect.
Many cases were consolidated into multidistrict litigation (MDL) to manage the high volume of claims. In a significant development, the Bair Hugger’s inventor, Dr. Scott Augustine, has publicly warned against its use in orthopedic surgeries.
If you developed a serious infection after joint replacement surgery, understanding your legal options is crucial. Bair Hugger Lawsuit offers more information, and Bair Hugger Infections Joint Replacement Lawsuits can connect you with specialized attorneys.
Understanding the Scientific Debate and Alternative Options
The controversy over the Bair Hugger is fueled by conflicting scientific evidence and the availability of alternative warming methods. This highlights the importance of informed consent and patient advocacy.
Conflicting Study Findings on Bacterial Contamination
The scientific community is divided on whether the Bair Hugger increases infection risk.
- Studies Supporting Safety: 3M points to over 170 studies and numerous clinical trials demonstrating the device’s safety. Some research, like a study finding no increased infection risk, found that the Bair Hugger actually decreased airborne bacteria near the patient, suggesting it may help keep surgical sites clean.
- Studies Raising Concerns: Other studies suggest the device’s waste heat can disrupt sterile airflow patterns in the operating room. Researchers have also raised concerns that the internal components of the warming unit could harbor bacteria, which could then be blown onto the patient.
This contradictory evidence makes it difficult for healthcare providers and patients to assess the true risk.
What is a bear hugger in medical terms compared to other methods?
The Bair Hugger is not the only patient warming technology available. Key alternatives include:
- Conductive Warming Systems: Devices like the HotDog Patient Warming System use resistive heating elements in pads that have direct contact with the patient’s skin. They do not circulate air, eliminating concerns about disrupting sterile airflow.
- Circulating Water Systems: These systems pump warm water through specialized blankets or pads. Like conductive systems, they transfer heat via direct contact and do not create air currents.
Each method presents a trade-off between warming effectiveness and potential risks. Forced-air warming is highly effective but faces infection risk allegations. Conductive and water-circulating systems avoid the airflow issue but may warm patients differently. As the debate continues, many hospitals are evaluating these alternatives, especially for high-risk procedures like joint replacements.
Understanding the Bair Hugger Medical Device
What is a bear hugger in medical terms refers to the Bair Hugger system – a forced-air warming device used in hospitals to prevent hypothermia during surgery. This medical device consists of a warming unit that blows heated, filtered air through disposable blankets placed on patients before, during, and after surgical procedures.
Quick Answer:
- Device Type: Forced-air warming (FAW) system for patient temperature management
- Primary Function: Prevents perioperative hypothermia by maintaining normal body temperature
- Components: Reusable warming unit, flexible hose, and single-use disposable blankets
- Usage: Used in over 80% of U.S. hospitals on more than 300 million patients since 1987
- Manufacturer: 3M Company (acquired from original inventor Dr. Scott Augustine)
The Bair Hugger is standard equipment in operating rooms but is also the subject of thousands of lawsuits alleging it increases infection risk during certain surgeries, particularly joint replacements.
What is the Bair Hugger System and How Does It Work?
The Bair Hugger system is a convective, forced-air warming (FAW) technology designed to maintain a patient’s body temperature during surgery.
The Core Function: Preventing Perioperative Hypothermia
Anesthesia and cool operating rooms can cause unintended perioperative hypothermia (a drop in core temperature), which is linked to:
- Increased blood loss and transfusion needs
- Higher surgical site infection risk
- Prolonged recovery and hospital stays
Maintaining normothermia throughout surgery helps reduce these complications.
Components and Mechanism of Action
- Reusable warming unit: Draws in room air, filters it (often via HEPA), and heats it to a controlled temperature.
- Flexible hose: Connects the unit to the blanket.
- Single-use disposable blankets: Designed with micro-perforations to distribute warmed air evenly.
Warm, filtered air flows through the hose into the blanket and exits through micro-perforations, creating a gentle layer of heat over the patient. This convective heat transfer helps maintain core temperature during the procedure.
The History and Widespread Adoption of Bair Hugger
From Invention to Industry Standard
Anesthesiologist Dr. Scott Augustine introduced forced-air warming in 1987 to address perioperative hypothermia. Adoption was rapid for its effectiveness and ease of use. Today, more than 80% of U.S. hospitals use the system, and it has warmed over 300 million patients worldwide. In 2010, the 3M Company acquired Arizant Inc., the manufacturer, integrating Bair Hugger into its medical portfolio. The story of 3M’s acquisition of the Bair Hugger manufacturer.
FDA Clearance and Regulatory Oversight
The Bair Hugger received initial FDA clearance in 1987 through the 510(k) process for devices substantially equivalent to existing products. The original 1987 FDA clearance document is publicly available. In 2018, there was a recall of 165,000 warming blankets due to a design defect that could prevent full inflation, potentially reducing warming effectiveness. This recall was unrelated to the infection allegations at the center of current litigation.
What is a Bear Hugger in Medical Terms? The Benefits vs. The Risks
When asking what is a bear hugger in medical terms, it’s important to weigh well-documented benefits of patient warming against allegations that have prompted widespread litigation.
The Scientific Case for Patient Warming
Maintaining normothermia during surgery improves outcomes:
- Reduced infections: Keeping patients warm can lower surgical site infection rates.
- Less blood loss and fewer transfusions
- Faster recovery and shorter hospital stays
3M cites 170+ studies supporting the Bair Hugger’s safety and effectiveness.
The Controversy: Allegations of Increased Infection Risk
Critics argue that forced-air currents may disrupt sterile airflow or circulate contaminants, potentially increasing infection risk in procedures such as joint replacements. These concerns focus on interference with laminar flow and possible contamination within warming units.
What is a bear hugger in medical terms regarding legal claims?
Thousands of patients have filed product liability lawsuits alleging design defects and failure to warn about infection risks. Many cases have been consolidated into multidistrict litigation (MDL), and inventor Dr. Scott Augustine has publicly cautioned against use in certain orthopedic surgeries. If you developed a serious infection after joint replacement surgery, learn more at Bair Hugger Lawsuit and connect with specialized attorneys via Bair Hugger Infections Joint Replacement Lawsuits.
Understanding the Scientific Debate and Alternative Options
The evidence on infection risk with forced-air warming is mixed, leading some providers to consider alternative warming methods for specific procedures.
Conflicting Study Findings on Bacterial Contamination
- Studies supporting safety: 3M references extensive research, including randomized trials. For example, a study finding no increased infection risk reported reduced airborne bacterial counts and sterile wound specimens when using FAW during long surgeries.
- Studies raising concerns: Other research suggests FAW may disrupt laminar airflow or that internal components could harbor contaminants, potentially elevating risk in ultra-clean surgical environments.
Variations in operating room design, measurement methods, and surgery types likely contribute to differing results.
What is a bear hugger in medical terms compared to other methods?
Alternatives to FAW include:
- Conductive (resistive) warming systems: e.g., HotDog Patient Warming System; pads transfer heat via direct contact, with no air movement.
- Circulating water systems: Warm water flows through blankets or pads, providing steady contact-based heat without airflow.
- Resistive warming blankets: Electrical elements woven into fabric deliver even heat.
Each approach balances warming efficiency, ease of use, and infection-control considerations. Discuss options with your surgical team, especially for high-risk procedures like joint replacements.
Free Confidential Case Evaluation
Complete the short form below to get an immediate FREE case review with an expert in your specific claim. Don't wait, your case could be time sensitive to file a claim.
Related Posts
Did a North Dakota product cause harm? Understand product liability, your rights, and how to take action for defects.
Get justice for clergy abuse. Find an expert Priest abuse lawyer to navigate complex laws and hold institutions accountable.
Diagnosed with meningioma after Depo-Provera? Understand potential Depo-Provera lawsuit settlements, risks, & how to claim compensation.
Uncover the truth about uber sexual assault cases. Learn about the alarming scale, Uber's accountability, and legal options for justice.
Facing wildfire losses? Discover the best wildfire lawsuit attorneys in California to fight for your full recovery and justice.
Exposed to Roundup & diagnosed with NHL? Discover how to sue Monsanto, understand eligibility, & seek compensation. Your guide to justice.