Understanding the Gummy Bear Implants Recall
The gummy bear implants recall refers to the recall of certain textured breast implants, primarily from manufacturer Allergan, due to a link to a rare form of cancer. Here’s a quick overview:
- What are “gummy bear” implants? This is a nickname for cohesive gel, form-stable silicone breast implants, known for maintaining their shape and firm yet soft feel.
- Why were some recalled? Certain textured breast implants, including some “gummy bear” types from Allergan, were recalled due to a higher risk of Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL).
- What is BIA-ALCL? It’s a rare T-cell lymphoma (a cancer of the immune system, not breast cancer) that can develop in the fluid and scar tissue around an implant.
- What are the symptoms? Watch for persistent swelling, pain, lumps, changes in breast size or shape, or fluid collection.
- What should I do? If you have these implants but no symptoms, the FDA doesn’t recommend removal due to surgical risks. However, it’s crucial to monitor for symptoms and consult your doctor with any concerns.
The term “gummy bear” refers to the unique, highly cohesive silicone gel that gives these implants their distinctive feel and shape retention. While popular, a specific type of textured surface on some of these implants, particularly Allergan’s BioCell, became the focus of a worldwide recall to address the confirmed link to BIA-ALCL.
What Are “Gummy Bear” Implants and Why Were They Recalled?
“Gummy bear” implants are a type of silicone breast implant filled with a highly cohesive, form-stable gel. Like the candy, they hold their shape even if the outer shell is compromised, a feature that made them popular for their natural look and feel.
Breast implants have either a smooth or textured outer surface. Smooth implants move freely within the breast pocket. Textured implants have a rougher surface designed to adhere to surrounding tissue, reducing the risk of shifting and a complication called capsular contracture.
The gummy bear implants recall centered on textured implants from Allergan that used their unique BioCell texturing. After a thorough investigation into Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL), the U.S. Food and Drug Administration (FDA) found a strong link between these specific implants and the rare cancer. In July 2019, the FDA requested Allergan issue a worldwide recall of its BioCell textured breast implants and tissue expanders. This was a Class I recall, the most serious type, used when a device can cause severe injury or death.
You can find details directly from The FDA’s official recall announcement.
The Specific Implants Included in the Recall
The recall specifically targeted Allergan’s BioCell textured products. It is crucial to know your implant’s manufacturer and type. The recall included both saline and silicone models with the BioCell texture:
- Natrelle Saline-Filled breast implants
- Natrelle Silicone-Filled breast implants
- Natrelle Inspira Silicone-Filled breast implants
- Natrelle 410 Highly Cohesive Anatomically Shaped Silicone-Filled breast implants
- Natrelle 133 Plus Tissue Expander
- Natrelle 133 Tissue Expander with Suture Tabs
The Science Behind the Risk: Textured vs. Smooth Surfaces
Scientists believe the BioCell texture itself is the problem. Created using a “lost salt” technique, its surface was rougher and more porous than other textured implants. This unique structure could:
- Trap bacteria: Leading to low-grade infections or a “biofilm” that causes chronic inflammation.
- Cause chronic irritation: The rough surface could physically chafe the surrounding tissue capsule.
- Overstimulate the immune system: This constant immune response might, in some individuals, lead to the genetic changes that cause BIA-ALCL.
The data was compelling: Allergan BioCell textured implants were found to be about six times more likely to cause BIA-ALCL than textured implants from other manufacturers. Despite making up less than 5% of implants sold in the U.S., they were linked to 86% of all reported BIA-ALCL cases where the implant type was known. This disparity strongly suggested the BioCell texture was a primary factor in developing this cancer.
The Link to Cancer: Understanding BIA-ALCL
The gummy bear implants recall is due to a serious, albeit rare, condition called Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL). It is crucial to understand that BIA-ALCL is not breast cancer. It is a rare type of T-cell lymphoma, a cancer of the immune system.
BIA-ALCL typically develops in the scar tissue capsule and fluid that naturally form around an implant, often appearing years after surgery (the average is 3 to 14 years). The link to Allergan’s implants is stark: by June 2023, these specific implants were associated with 1,264 illnesses and 63 deaths globally. The overwhelming majority of BIA-ALCL cases where the implant manufacturer was known were linked to Allergan.
If you are concerned about your implants or wish to explore your legal options, we can help you get answers.
More info on Breast Implant Lawsuits
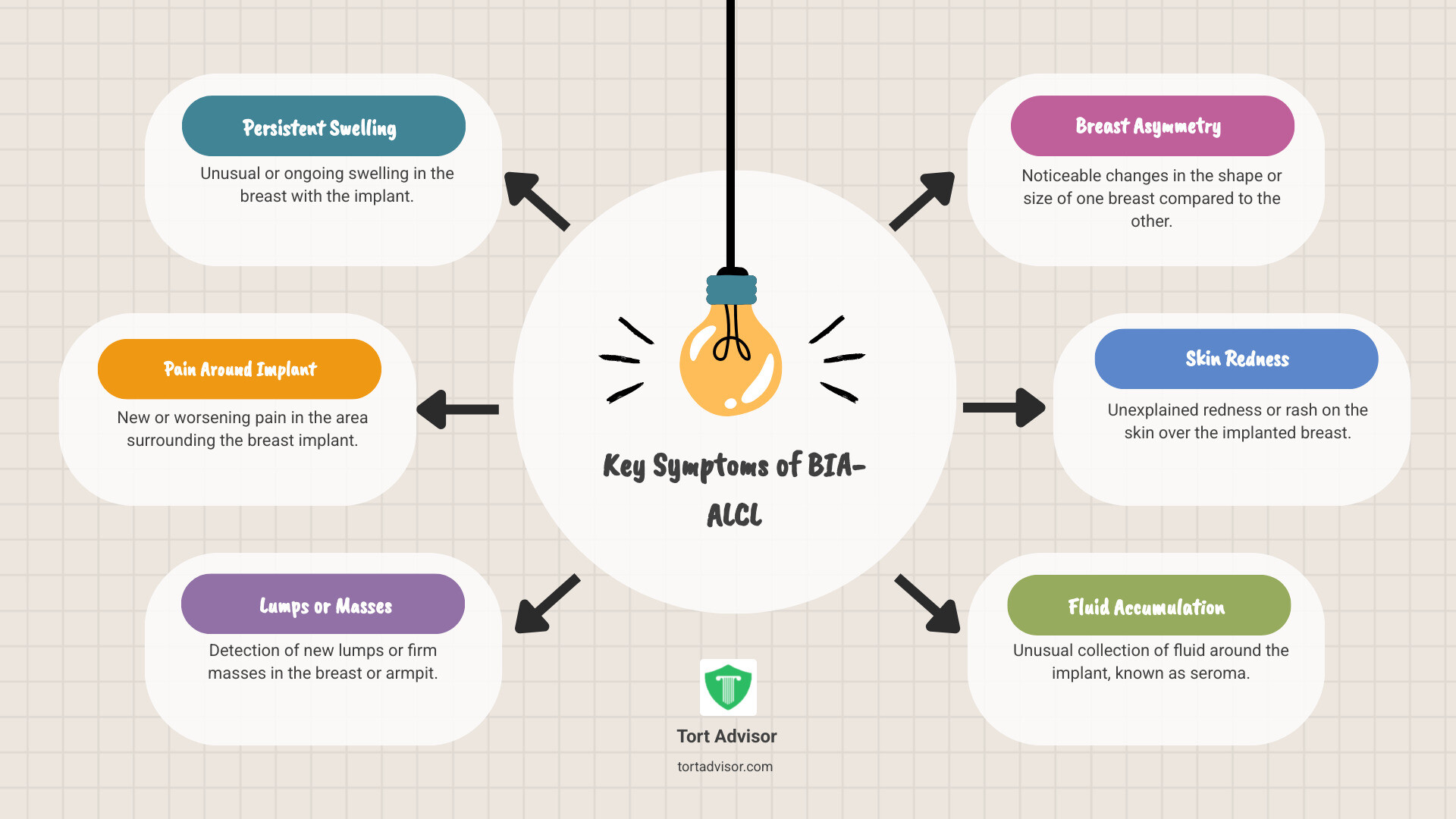
Key Symptoms of BIA-ALCL
Being aware of the symptoms is critical, as early detection is key. Symptoms are usually caused by fluid buildup (seroma) or a mass forming around the implant. Be watchful for:
- Persistent swelling or a sudden increase in breast size
- Pain in or around the implant
- New lumps or masses in the breast or armpit
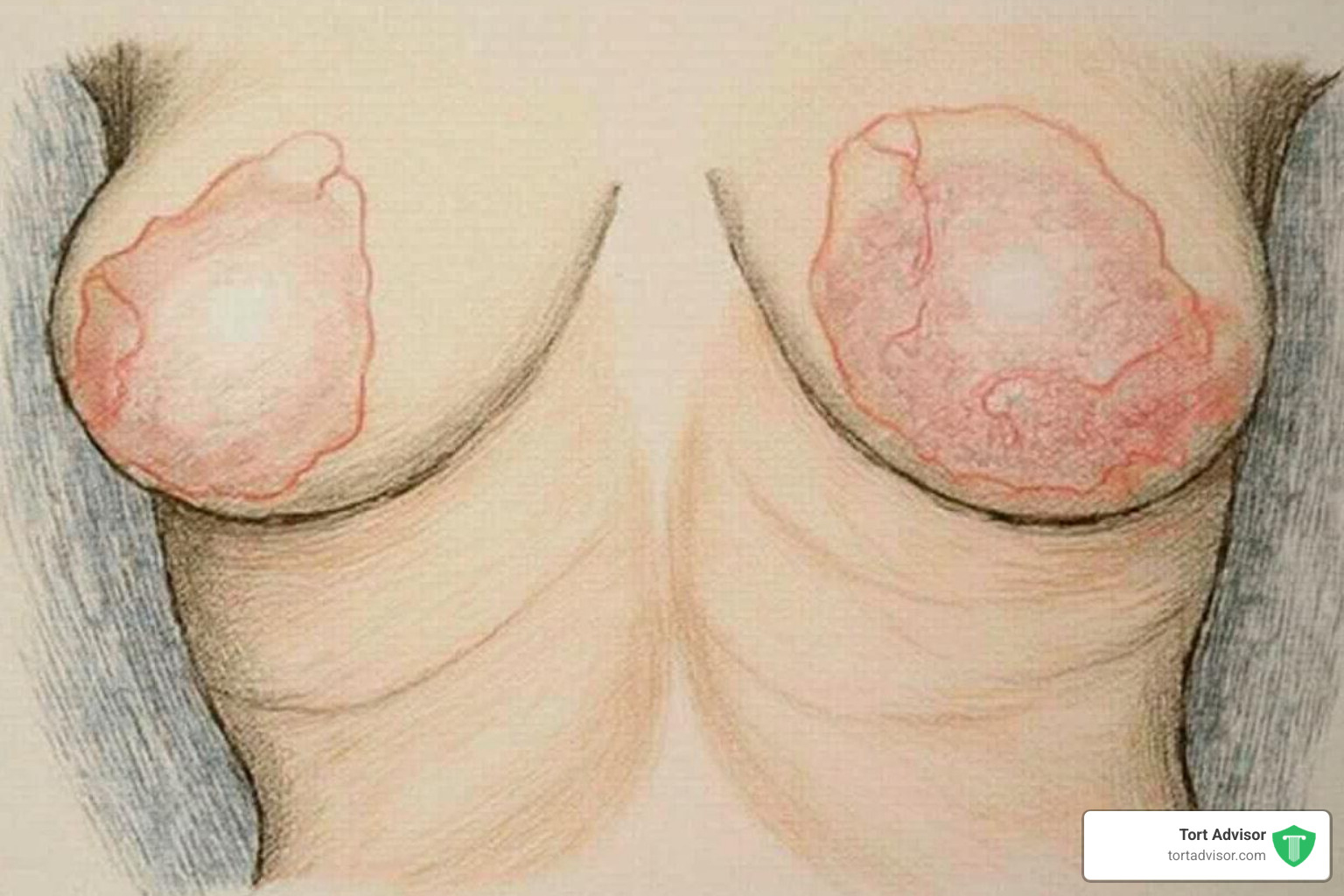
- Breast asymmetry (a change in breast shape or size)
- Skin redness or a rash over the breast
- Fluid accumulation
If you experience any of these symptoms, contact your doctor promptly.
How is BIA-ALCL Diagnosed and Treated?
If BIA-ALCL is suspected, diagnosis typically involves:
- Imaging: An ultrasound or MRI is used to visualize fluid or masses around the implant.
- Fluid and Tissue Testing: If fluid is present, a sample is collected and tested for specific cancer markers (like CD30). A biopsy is performed on any solid mass.
The good news is that BIA-ALCL is highly treatable, especially when caught early. The standard treatment is surgery to remove the breast implant and the entire surrounding scar tissue capsule (a total capsulectomy). For most patients with early-stage disease, this surgery is curative. In rarer, more advanced cases where the cancer has spread, chemotherapy or radiation therapy may be necessary.
Navigating the Gummy Bear Implants Recall: What Patients Need to Do
Hearing about the gummy bear implants recall can be alarming. This section provides clear, actionable advice based on official recommendations to help you steer the situation.
The U.S. Food and Drug Administration (FDA) advises that if you have recalled Allergan BioCell textured implants but are not experiencing any symptoms, you do not need to have them removed. This is because any surgery has inherent risks that may outweigh the very small chance of developing BIA-ALCL. However, vigilant monitoring for symptoms is essential. If you notice any changes, contact your healthcare provider immediately.
What to Do if You Have Recalled Implants but No Symptoms
If you have confirmed you have recalled implants but feel fine, here are the recommended steps:
- Know Your Implant Type: If you are unsure, request your operative report from your surgeon’s office. It will list the manufacturer, model, and type of your implants.
- Learn the Symptoms of BIA-ALCL: Be aware of what to look for: persistent swelling, pain, lumps, asymmetry, or redness.
- Perform Regular Self-Exams: Routinely check your breasts for any unusual changes.
- Maintain Check-ups: Continue with your regular medical appointments and recommended breast screenings.
- Discuss Your Concerns: While prophylactic removal isn’t recommended, the anxiety of having recalled implants is a valid concern. Discuss your feelings and options with your surgeon.
Legal Options for Those Affected by the Gummy Bear Implants Recall
Individuals diagnosed with BIA-ALCL, or those who required removal surgery due to symptoms or severe anxiety related to the recall, may have legal options. These product liability lawsuits often allege that Allergan failed to adequately warn patients and doctors about the risks associated with their BioCell textured implants.
Many of these federal cases have been consolidated into a Multi-District Litigation (MDL) in New Jersey to streamline the process. As of September 2024, over 1,200 lawsuits were pending in the Allergan breast implant MDL. Compensation may be sought for:
- Medical expenses for diagnosis, treatment, and care
- Lost wages from time off work
- Pain and suffering, including physical discomfort and emotional distress
Allergan offered a limited warranty program, but it often falls short of covering the full costs. Navigating the legal system can be complex. Tort Advisor connects people with top-rated specialty attorneys across the nation who have a proven history in product liability cases. We can help you understand your rights and determine if a claim is right for you.
Details on the Allergan Textured Implant Lawsuit Settlement
Broader Risks and Considerations for All Breast Implants
While the gummy bear implants recall focused on a specific risk with textured implants, it’s important to understand that all breast implants carry potential risks and are not considered lifetime devices. Future surgeries for replacement or removal are common.
One widely discussed topic is Breast Implant Illness (BII), a term for a collection of systemic symptoms that some women with implants report. While not yet a formal medical diagnosis, these symptoms can include fatigue, “brain fog,” joint and muscle pain, anxiety, hair loss, and skin rashes. Many patients report significant improvement after their implants are removed.
Another common complication is capsular contracture, where the scar tissue around the implant tightens and hardens, potentially causing pain and a distorted appearance.
Other potential risks include:
- Implant rupture or deflation: Silicone implants can rupture “silently” without obvious signs, which is why the FDA recommends periodic MRI screenings.
- Additional surgeries: Revision surgeries may be needed due to rupture, capsular contracture, or aesthetic concerns.
- Infection: As with any surgery, infection is a risk.
- Changes in nipple sensation
- Aesthetic concerns like wrinkling or asymmetry.
What to Know Before Considering Breast Implants
If you are considering breast implants, it is vital to be fully informed.
- Informed Consent is Key: Your surgeon should explain all risks, benefits, and alternatives. Ask questions until you feel confident.
- Implants Are Not Lifetime Devices: Plan for the likelihood of future surgeries for removal or replacement.
- Discuss All Risks: Have a candid conversation with your surgeon about BIA-ALCL, BII, and other potential complications.
- Choose Your Implant Carefully: Work with your surgeon to select the best implant type and surface for your goals, keeping safety information in mind.
- Understand Monitoring Requirements: Silicone implants require regular MRI or ultrasound screenings to check for silent ruptures, typically starting 5-6 years post-surgery.
- Consider Long-Term Costs: Factor in the potential future expenses for monitoring and additional surgeries.
Does Insurance Cover Removal of Recalled Implants?
Insurance coverage for implant removal (explantation) depends on your policy and the reason for removal.
- Medically Necessary Removal: Coverage is more likely if removal is deemed medically necessary. This typically includes a diagnosis of BIA-ALCL, a symptomatic rupture, or severe capsular contracture.
- Asymptomatic or Elective Removal: If you have no symptoms and want the implants removed due to anxiety or for cosmetic reasons, insurance is unlikely to cover the procedure.
It is essential to contact your insurance provider directly to understand your specific policy. If your doctor recommends removal for medical reasons, ensure they provide clear documentation to support the claim.
Conclusion
The gummy bear implants recall involving Allergan’s BioCell textured implants has been a critical chapter in medical device safety, highlighting the risk of Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL).
We’ve covered what these implants are, why they were recalled, and the key symptoms of BIA-ALCL to monitor for. While the FDA does not recommend removal for asymptomatic patients, staying vigilant is crucial. We’ve also explored the broader landscape of implant risks, including Breast Implant Illness (BII) and the fact that implants are not lifetime devices.
For those harmed by the recall, legal options exist through product liability lawsuits to seek compensation and hold manufacturers accountable. Understanding your rights is the first step toward justice and peace of mind.
At Tort Advisor, our mission is to connect you with elite specialty attorneys who have a proven track record in complex medical device litigation. If the gummy bear implants recall has affected your life, let us help you explore your options and connect you with the legal support you deserve.
Learn more about your options in a Personal Injury Lawsuit
Free Confidential Case Evaluation
Complete the short form below to get an immediate FREE case review with an expert in your specific claim. Don't wait, your case could be time sensitive to file a claim.
Related Posts
Did a North Dakota product cause harm? Understand product liability, your rights, and how to take action for defects.
Get justice for clergy abuse. Find an expert Priest abuse lawyer to navigate complex laws and hold institutions accountable.
Diagnosed with meningioma after Depo-Provera? Understand potential Depo-Provera lawsuit settlements, risks, & how to claim compensation.
Uncover the truth about uber sexual assault cases. Learn about the alarming scale, Uber's accountability, and legal options for justice.
Facing wildfire losses? Discover the best wildfire lawsuit attorneys in California to fight for your full recovery and justice.
Exposed to Roundup & diagnosed with NHL? Discover how to sue Monsanto, understand eligibility, & seek compensation. Your guide to justice.