Recent Research Links Depo-Provera to Increased Brain Tumor Risk
Does Depo-Provera cause brain tumors? Recent scientific studies suggest that prolonged use of Depo-Provera may significantly increase the risk of developing brain tumors, specifically meningiomas.
Quick Answer:
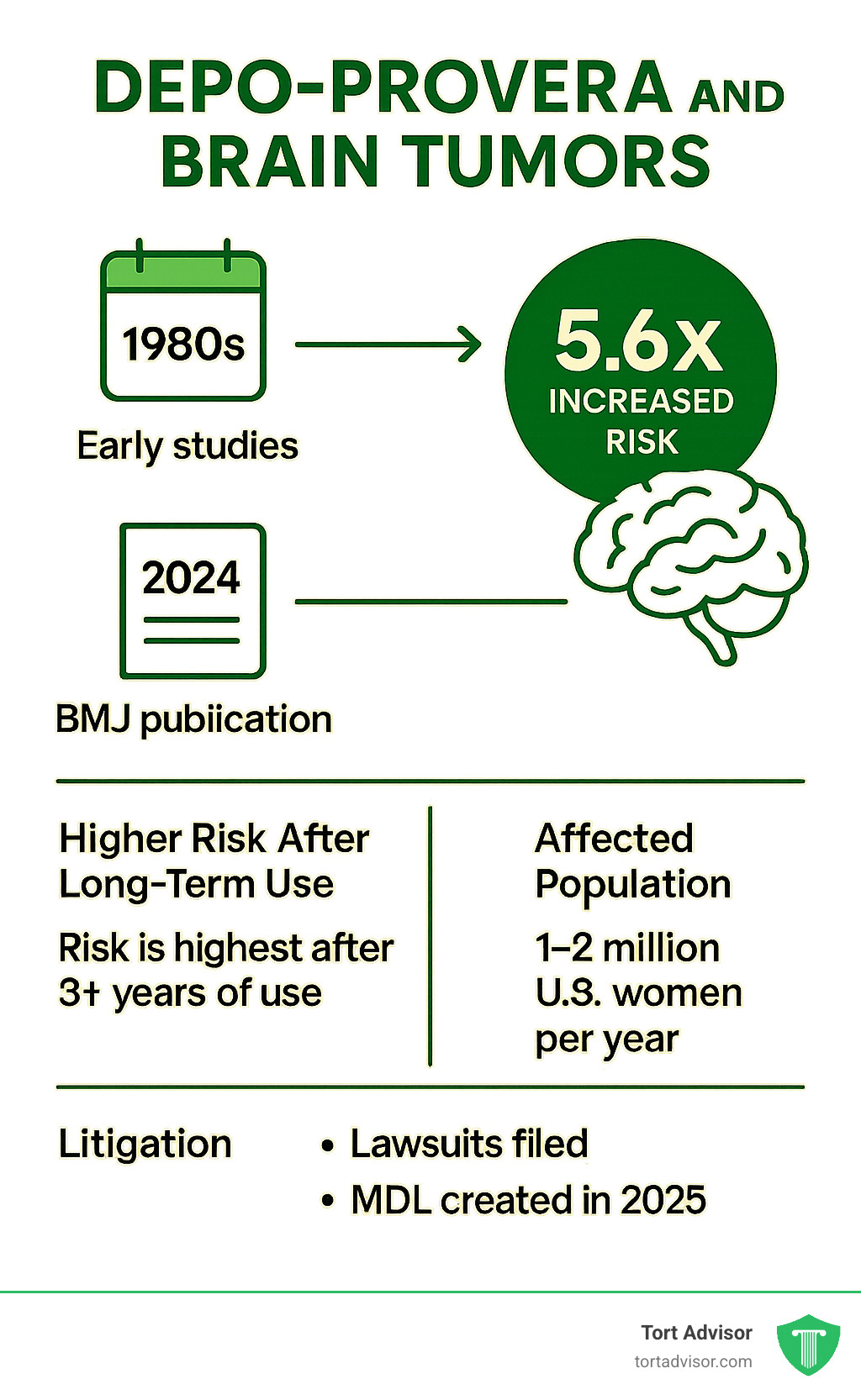
- 5.6x increased risk of meningiomas with long-term Depo-Provera use (BMJ 2024 study)
- Risk increases with duration – highest risk after 3+ years of use
- Only injectable forms show this link, not oral versions
- Absolute risk remains low but affects millions of users worldwide
A groundbreaking study published in the British Medical Journal in March 2024 found that women using Depo-Provera for one year or longer had a 5.55-fold increased risk of developing intracranial meningiomas compared to non-users. This finding was supported by a large U.S. insurance database study published in September 2024, which showed a 53% increased risk overall, rising to 2.5-fold for women with exposure durations over three years.
The research is particularly concerning because approximately 1 to 2 million women in the United States use Depo-Provera annually, and about 20% of all U.S. women have used it at some point in their lifetime. While meningiomas are typically benign, they can cause serious symptoms including headaches, vision problems, and seizures, often requiring surgical removal.
What Is Depo-Provera and How Is It Used?
Depo-Provera is the brand name for medroxyprogesterone acetate (MPA), a synthetic hormone given as a shot every three months to prevent pregnancy. The FDA approved it for birth control in October 1992, though it had been used worldwide for nearly 60 years.
Each injection contains 150 mg of medroxyprogesterone acetate, which prevents ovulation, thickens cervical mucus, and thins the uterine lining. This makes it over 99% effective at preventing pregnancy when administered on schedule.
The usage statistics are staggering: up to 25% of U.S. women aged 15-49 who have had sex use MPA at some point. Between 2015-2019, nearly one in four sexually active women reported using Depo-Provera, with particularly high usage rates among Black women (41.2%) and Hispanic women (27.2%).
Concerningly, the FDA has already issued its strongest possible warning – a black box warning – for Depo-Provera because of bone density loss. The official label says the drug shouldn’t be used long-term for more than two years unless no other acceptable birth control options exist.
For more comprehensive information about other serious side effects, you can read about Depo-Provera Severe Side Effects.
Depo-SubQ vs Intramuscular vs Oral Forms
Not all forms of medroxyprogesterone acetate carry the same brain tumor risk. Only the injectable forms are linked to increased meningioma risk.
Depo-Provera (the regular shot) is the standard 150 mg injection given deep into muscle tissue every 12 weeks. This is most strongly linked to brain tumor risk.
Depo-SubQ Provera 104 is a lower-dose version (104 mg) injected under the skin every 12-14 weeks. It’s approved for both birth control and treating endometriosis pain. Even at the lower dose, brain tumor concerns likely apply since it’s still injectable.
Oral MPA comes in pill form for treating various conditions. Crucially, studies show that oral forms of MPA are not associated with increased meningioma risk. The brain tumor risk appears specific to injectable versions.
The convenience of quarterly injections has made Depo-Provera popular, but this convenience comes with serious trade-offs. Unlike pills you can stop taking immediately, the long-acting injection means side effects – including brain tumor risk – can’t be immediately reversed.
Understanding Brain Tumors, Especially Meningiomas
When discussing does Depo-Provera cause brain tumors, we’re specifically talking about meningiomas – tumors that develop in the protective membranes wrapping around your brain and spinal cord. These tumors make up about one-third of all brain tumors and roughly 40% of all central nervous system tumors.
Even though most meningiomas are technically benign (non-cancerous), they can cause serious problems. When a tumor grows inside your skull, there’s no extra room. The pressure affects everything from vision to cognitive function.
Symptoms often develop gradually, making them dangerous to ignore. Persistent headaches are often the first sign, especially morning headaches that don’t respond to typical pain relievers. Vision problems like double or blurry vision can develop, along with hearing loss or constant ringing in the ears.
Many patients experience memory loss and cognitive difficulties that can be mistaken for stress or aging. Seizures can occur suddenly, even in people who’ve never had them. Other warning signs include speech problems, loss of smell, limb weakness, and coordination problems.
Treatment depends on tumor size, location, and growth rate. Small, slow-growing meningiomas might only need monitoring with regular MRI scans. But larger or symptomatic tumors often require surgical removal through craniotomy.
Brain surgery carries significant risks including infection, bleeding, and neurological complications. Recovery can involve months of rehabilitation, with treatment costs exceeding $700,000 before insurance. Even “successful” surgeries can leave patients with permanent changes to personality, memory, or physical abilities.
For detailed information about meningioma basics, the National Cancer Institute provides comprehensive resources.
Why Are Women More Affected?
Meningiomas are three times more common in women than men after puberty, revealing important biological connections. The key lies in progesterone receptors – between 38-88% of meningiomas have these receptors, making them incredibly sensitive to hormonal influences.
This hormonal connection explains why meningiomas sometimes grow during pregnancy when progesterone levels naturally skyrocket. It also helps us understand why synthetic progestins like those in Depo-Provera might fuel tumor growth.
Encouragingly, some meningiomas actually shrink when progesterone levels drop, such as after menopause or when hormone therapy is stopped. This reversibility supports a real causal relationship between Depo-Provera and meningioma development.
Does Depo-Provera Cause Brain Tumors? Latest Scientific Evidence
The question “does Depo-Provera cause brain tumors” has been answered with alarming clarity by recent groundbreaking research. The evidence is overwhelming and consistent across multiple large-scale studies.
The most significant breakthrough came from a landmark study published in the British Medical Journal in March 2024. French researchers analyzed data from over 100,000 women between 2009 and 2018, comparing 18,061 women who underwent meningioma surgery with 90,305 matched controls. The results: women who used Depo-Provera for one year or longer had a 5.55-fold increased risk of developing brain tumors.
A massive U.S. study published in the journal Cancers in September 2024 confirmed these findings. This research analyzed 117,503 meningioma cases and over one million matched controls from a large insurance database, showing remarkably consistent results with the French study.
The U.S. research showed women using injectable MPA had a 53% increased risk of developing meningiomas overall. For women who used Depo-Provera for more than three years, the risk jumped to 2.5 times higher than non-users. When researchers looked specifically at brain tumors, they found a 68% increased risk.
The fact that two independent studies in different countries with different healthcare systems reached nearly identical conclusions makes the evidence particularly compelling. This consistency strongly supports the conclusion that Depo-Provera does cause brain tumors.
For detailed scientific research on progestogen risk, you can review the original BMJ study.
2024 BMJ Findings: Does Depo-Provera Cause Brain Tumors?
The BMJ study represents the gold standard for investigating whether Depo-Provera causes brain tumors. The researchers used a national case-control design following women for an average of 6.8 years.
The study focused on meningiomas requiring surgical intervention – not small, incidental tumors, but significant ones causing real symptoms and requiring brain surgery. The 5.55-fold increased risk is medically significant, comparable to certain smoking-lung cancer relationships.
Importantly, the research design controlled for other risk factors including age, medications, and medical history. This means the increased risk appears directly related to Depo-Provera use.
Dose-Response: How Duration and Dosage Matter
One of the most convincing pieces of evidence is the clear dose-response relationship. The longer women used Depo-Provera, the higher their risk became.
The U.S. study showed this progression clearly: women with any exposure had a 53% increased risk, those using it for 1-3 years had moderate risk increase, but women using Depo-Provera for more than 3 years faced a 2.5-fold increased risk.
This dose-response relationship is what epidemiologists call a “smoking gun” for causation. The number of injections also matters significantly, with highest risk in those receiving many injections over several years.
Injectable vs Oral MPA and Other Hormonal Birth Control
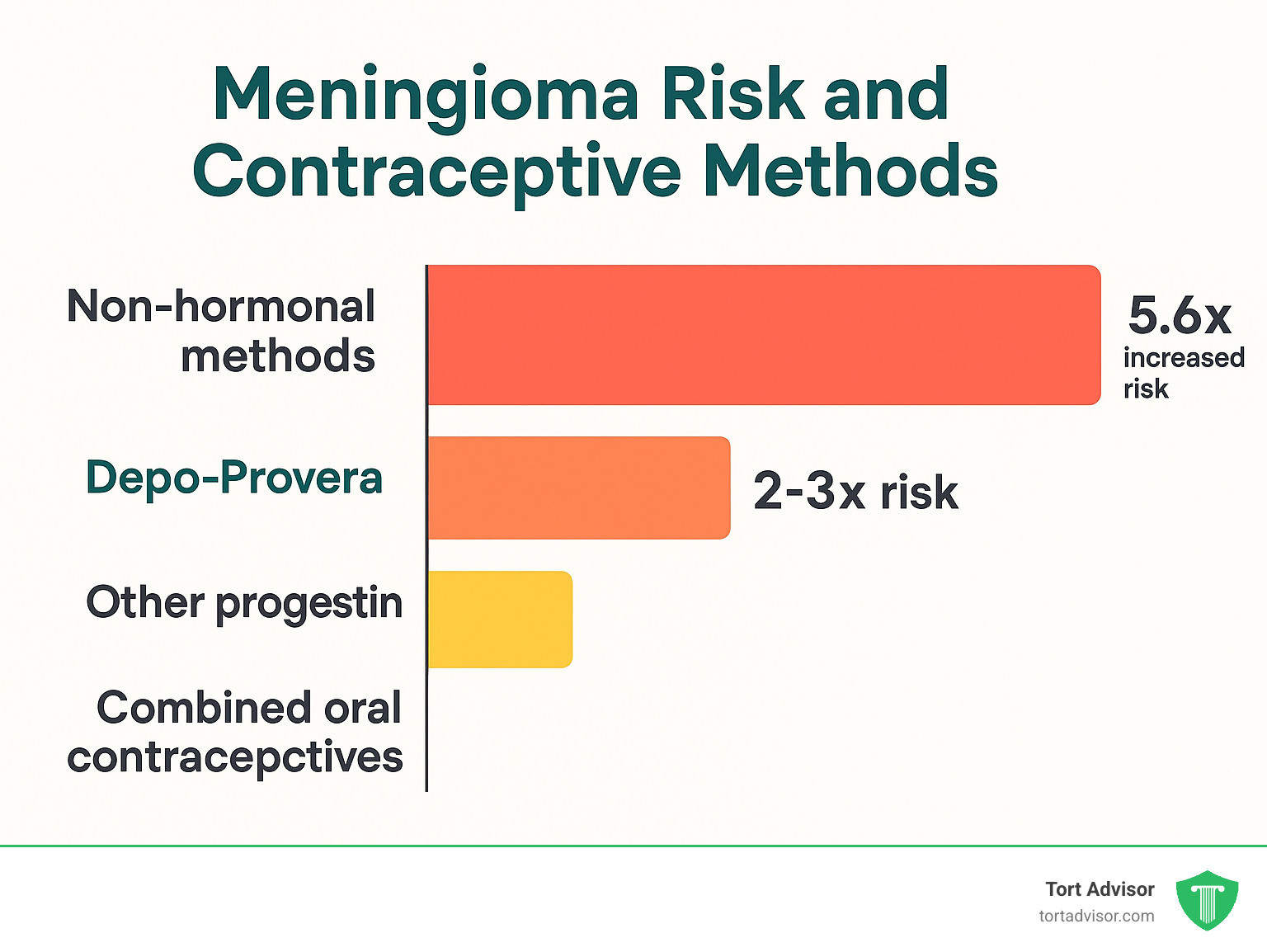
Only injectable forms of medroxyprogesterone acetate are associated with increased brain tumor risk. Oral MPA showed no significant association with meningiomas.
This distinction is crucial because it shows that delivery method and resulting hormone levels matter enormously. Injectable MPA creates much higher and more sustained hormone levels than oral forms.
Combined oral contraceptives generally show minimal or no increased meningioma risk. Levonorgestrel-releasing IUDs present a more complex picture with less clear evidence. Non-hormonal methods like copper IUDs show no increased brain tumor risk.
Biological Mechanisms Behind the Link
When examining does Depo-Provera cause brain tumors, scientists have identified clear biological pathways explaining this connection. The story starts with progesterone receptors – between 38% and 88% of meningiomas have these receptors, making them primed to respond to synthetic hormones like those in Depo-Provera.
When medroxyprogesterone acetate binds to these receptors, it triggers several concerning processes:
Cell proliferation occurs when normal cell division stop signals get overridden. Cells start dividing more frequently than they should, potentially leading to tumor formation.
The hormone promotes angiogenesis – new blood vessel formation. While this sounds harmless, tumors need blood supply to grow beyond a certain size. Synthetic progesterone essentially helps feed developing tumors.
Most troubling is interference with apoptosis – the body’s natural process of eliminating damaged cells. When progesterone signaling disrupts this quality control system, abnormal cells that should die instead stick around and multiply.
What makes Depo-Provera particularly concerning is the sustained high levels of synthetic progesterone it creates. Unlike natural hormone fluctuations, Depo-Provera maintains consistently liftd hormone levels for months. This constant stimulation may overwhelm normal regulatory mechanisms.
The injection delivers such high hormone concentrations that it can suppress natural hormone production for up to 18 months after the last shot. During this period, the meninges are bathed in artificial hormones at levels far exceeding natural intentions.
This biological understanding explains why only injectable forms show increased brain tumor risk – oral forms create much lower, more variable hormone levels that don’t trigger the same dangerous cellular responses.
For comprehensive study details on hormone receptors, this research provides detailed pathway information.
What Should Current or Former Users Do Now?
If you’re currently using or have used Depo-Provera, don’t panic. While “does Depo-Provera cause brain tumors” has been answered with concerning evidence, the absolute numbers remain relatively low. What matters now is taking informed action.
Most important: Don’t make sudden medication changes without consulting your doctor. Stopping Depo-Provera abruptly can leave you without contraceptive protection.
If currently using Depo-Provera, discuss your individual risk factors with your healthcare provider. Your age, duration of use, and family history all influence your personal risk level. Together, weigh continued use benefits against newly finded risks.
Consider non-hormonal alternatives like copper IUDs, barrier methods, or permanent sterilization. These don’t carry meningioma risk, though each has its own benefits and drawbacks.
For former users, stay vigilant about symptoms while documenting your exposure history. Calculate total exposure by determining injection count and time period. This information is valuable for medical monitoring and potential legal claims.
Keep detailed medical records of your Depo-Provera usage – dates, locations, healthcare providers involved. This documentation could prove crucial for symptom development or legal options.
For comprehensive legal information, visit our Depo-Provera Lawsuit page.
Monitoring Symptoms of Meningioma
Recognizing meningioma warning signs can be life-saving. Brain tumor symptoms often start subtle and gradually worsen, making them easy to dismiss.
Headache patterns are often the first clue. Meningioma-related headaches tend to be worse in the morning, may wake you from sleep, and don’t respond well to over-the-counter medications. They typically worsen progressively over weeks or months.
Vision changes occur because growing tumors press on optic nerves. You might notice blurred vision, double vision, or peripheral vision loss. These changes are usually gradual.
Memory issues and cognitive changes affect daily life subtly. You might forget appointments, have trouble concentrating, or notice personality changes that others comment on. These symptoms are often dismissed as aging or stress.
Seizures in adults who’ve never had them should always be taken seriously, especially with Depo-Provera history.
Other symptoms include weakness on one body side, speech difficulties, hearing loss, or smell loss. If you experience any combination with Depo-Provera history, seek medical evaluation promptly.
Can Stopping Depo-Provera Reduce Risk?
Stopping Depo-Provera might reduce meningioma risk over time. A Pennsylvania case series found that among women who stopped Depo-Provera after developing meningiomas, about half experienced tumor shrinkage.
The biological mechanism makes sense – since many meningiomas have progesterone receptors, removing synthetic progesterone can potentially slow tumor growth or cause shrinkage.
However, not all meningiomas will shrink after stopping, and some may continue growing. Effects can take months or years to become apparent, with individual responses varying significantly.
Legal Options and Filing Deadlines
The findy of Depo-Provera’s brain tumor link has sparked significant legal action. In February 2025, federal Multidistrict Litigation (MDL) was established in Northern District of Florida.
You may be eligible for compensation if you received at least two Depo-Provera injections and were later diagnosed with meningioma. Legal theory centers on allegations that Pfizer failed to adequately warn about meningioma risk despite knowledge from earlier studies.
Compensation can include medical expenses, lost wages, diminished earning capacity, pain and suffering, and ongoing medical monitoring costs. Given that brain tumor treatment can exceed $700,000 before insurance, these damages can be substantial.
Filing deadlines are crucial and vary by state. Most statutes of limitations range from 2-3 years from diagnosis or findy date. Having experienced legal representation is essential for pharmaceutical litigation complexity.
Frequently Asked Questions about Depo-Provera & Brain Tumors
Does Depo-Provera cause brain tumors in short-term users?
The research brings relief for brief users. Short-term use of less than one year doesn’t significantly increase brain tumor risk. Concerning findings focus on women receiving injections for longer periods.
However, some researchers suggest six months of use might begin to slightly increase risk – though much smaller than long-term use increases. Does Depo-Provera cause brain tumors appears primarily a concern for extended use.
Are meningiomas cancerous or benign?
Most meningiomas (85-90%) are technically “benign,” meaning they don’t spread like typical cancers. But don’t let “benign” fool you – benign doesn’t mean safe for brain tumors.
These growths cause serious problems because your brain sits inside a rigid skull with no extra room. Meningiomas can cause persistent headaches, vision problems, seizures, and memory issues. Many require complex surgical removal with significant risks.
About 10-15% are classified as atypical or malignant, which are more aggressive. Even benign ones can occasionally transform into dangerous forms over time.
Who is most at risk when using Depo-Provera?
Duration of use stands out as the strongest risk factor – women using Depo-Provera for more than three years face highest meningioma risk.
Age plays a significant role. Two-thirds of Depo-Provera users who developed meningiomas were over 45 years old. Risk increases with age, though younger women aren’t immune.
Black and Hispanic women use Depo-Provera at significantly higher rates – 41.2% and 27.2% respectively. While race isn’t a biological risk factor, these higher usage rates mean more women in these communities face potential exposure risks.
Conclusion
The scientific evidence definitively answers: does Depo-Provera cause brain tumors? Yes. Research shows a clear 5.6-fold increased risk of meningiomas in women using this contraceptive injection long-term.
This isn’t just statistics – it’s a real concern affecting millions worldwide. When up to 25% of sexually active U.S. women have used Depo-Provera, we’re discussing significant numbers of people at increased brain tumor risk.
The most troubling aspect isn’t just the increased risk, but that women weren’t properly warned for decades. Many made reproductive health decisions without knowing this potential consequence. That’s not informed consent – that’s system failure.
For current users, the path forward involves honest healthcare provider conversations about alternatives. Effective non-hormonal options exist, from copper IUDs to barrier methods. The key is making informed choices based on complete risk-benefit information.
For former users, staying alert to symptoms while understanding legal rights becomes important. Brain tumors can develop years after exposure, so awareness of warning signs like persistent headaches, vision changes, or cognitive problems remains crucial.
At Tort Advisor, we connect clients with top-rated specialty attorneys with proven pharmaceutical litigation track records. Our network includes experienced lawyers across all states who understand these cases’ complexities and can evaluate your potential claim.
The legal landscape is evolving rapidly. With federal multidistrict litigation establishment in 2025, courts recognize these claims’ seriousness. Legal action serves two purposes – providing compensation paths for medical expenses, lost wages, and pain and suffering, while sending clear messages that pharmaceutical companies must be transparent about medication risks.
We work exclusively with highly skilled attorneys with proven results because facing brain tumor diagnosis is overwhelming enough without inadequate legal representation concerns. Our network attorneys have resources and expertise to take on major pharmaceutical companies and fight for deserved compensation.
The evidence about Depo-Provera causing brain tumors represents more than scientific findings – it’s a call for better women’s health protection and honest medication risk communication. No woman should find years later that a trusted medication put her at serious risk.
If you or someone you love used Depo-Provera and developed meningioma, you don’t have to steer this challenge alone. Legal action provides potential compensation while helping prevent similar situations affecting other women.
For more information about legal options and connecting with qualified attorneys, visit our Depo-Provera Lawsuits page.
The research is clear, risks are real, and legal options are available. The question now is what you’ll do with this information to protect yourself and hold those responsible accountable.
Free Confidential Case Evaluation
Complete the short form below to get an immediate FREE case review with an expert in your specific claim. Don't wait, your case could be time sensitive to file a claim.
Related Posts
Learn how to qualify for an Uber sexual assault lawsuit: MDL 3084 criteria, evidence, deadlines & compensation steps.
Consult cosmetic injury lawyers for botched procedures. Learn negligence proof, damages, & claims against surgeons today!
Understand your rights after a California wildfire. Learn how to file a wildfire lawsuit California, claim compensation, and hold utilities accountable.
Find a top Mississippi accident lawyer. Get immediate steps, state laws, compensation tactics & max recovery after your crash.
Discover New Jersey disability benefits: TDI, FLI, SSDI, SSI rates, eligibility, applications & appeals for 2025-2026.
Hire a Depo-Provera lawsuit attorney now. Fight Pfizer for meningioma risks from injections. Free consult, MDL updates & settlements up to $1.5M.