

What You Need to Know About the Growing Depo-Provera Litigation
Depo provera lawsuit news has been a major legal topic in 2024 and 2025, with over 1,200 federal lawsuits alleging the popular birth control shot causes serious brain tumors. Here’s a quick summary:
Quick Summary of Current Status:
- Over 1,200 lawsuits are consolidated in federal MDL 3140 in the Northern District of Florida.
- Pfizer filed a motion for summary judgment, arguing federal law shields them from state-level claims.
- A 2024 BMJ study linked Depo-Provera use of over a year to a 5.6 times higher risk of meningioma brain tumors.
- Five bellwether cases have been selected for early trials to test legal arguments.
- Estimated settlements may range from $100,000 to over $1 million, depending on injury severity.
- Most states allow 2-3 years after diagnosis to file a claim.
The central allegation is that Pfizer knew or should have known about the link between Depo-Provera and brain tumors but failed to provide adequate warnings. While European and Canadian regulators required warnings years ago, the U.S. label still lacks specific information on meningioma risk.
Pfizer claims the FDA rejected their proposed label changes (a defense known as “federal preemption”). However, plaintiffs argue the company withheld critical evidence from regulators. This legal battle will determine if thousands of claims can proceed.
My name is Mason Arnao. My background is in analyzing complex systems, and I apply those skills to explain intricate legal topics like the depo provera lawsuit news. My goal is to provide clear, actionable information for people facing difficult decisions.
Latest Depo Provera Lawsuit News and MDL Updates
Major developments in the depo provera lawsuit news are unfolding rapidly. The litigation is centered around Multidistrict Litigation (MDL) 3140, consolidated in the Northern District of Florida under Judge M. Casey Rodgers. An MDL gathers similar lawsuits into one court for efficient pretrial proceedings, preventing contradictory rulings and saving resources.
As of October 2025, the MDL includes over 1,200 lawsuits, a number that has been climbing weekly. To manage the litigation, the court has selected five bellwether cases. These initial trials serve as test cases, giving both sides a preview of how juries might respond to the evidence and influencing potential settlement negotiations for the entire MDL.
Judge Rodgers has also issued Case Management Orders (CMOs) to set rules and timelines. A key deadline is July 2025 for legal teams to submit Plaintiff Proof of Use Questionnaires. These forms verify that each plaintiff used Depo-Provera (or an authorized generic) and subsequently developed a meningioma, ensuring all cases in the MDL meet basic qualifications.
For the most current updates on how this litigation is progressing, check out our dedicated page: Depo-Provera Lawsuit Updates 2025.
Current Status of the Federal Litigation
The MDL consolidation centralizes all federal Depo-Provera lawsuits for pretrial proceedings. However, the litigation is not confined to federal court. Judge Rodgers is actively coordinating with state courts, where filings are also substantial. As of October 2025, there are 71 cases in New York, 19 in California, and a dozen more across several other states.
This nationwide spread of cases highlights that women across the country are reporting similar injuries. The coordination between state and federal courts aims to handle these claims efficiently and consistently.
Why the Number of Lawsuits is Rapidly Increasing
Three main factors are driving the surge in depo provera lawsuit news and filings:
- New Scientific Studies: Compelling evidence, including a 2024 study in The British Medical Journal, has linked Depo-Provera to a 5.6-fold increased risk of meningiomas. This data gives women and their attorneys a strong scientific basis for their claims.
- Increased Public Awareness: News coverage and legal advertising have helped women connect their symptoms—such as unexplained headaches, vision problems, or seizures—to their past use of the birth control shot.
- Mass Tort Structure: The formation of an MDL creates momentum. It lowers the barrier to filing a claim, as specialized legal teams develop efficient systems to evaluate and handle cases, providing a clearer path forward for individuals suing a large corporation.
As awareness spreads, this upward trend in filings is expected to continue.
Want to understand where this litigation stands legally? Visit: What’s the Legal Status of the Depo-Provera Lawsuits?.
Pfizer’s High-Stakes Legal Battle: The Preemption Defense
Pfizer has responded to the growing number of lawsuits by filing a motion for summary judgment, a legal maneuver to have key claims dismissed before trial. If successful, this could halt a significant portion of the litigation.
Pfizer’s defense is built on federal preemption. This legal principle holds that federal law supersedes state law when they conflict. In pharmaceutical litigation, this can shield a company from state-level failure-to-warn lawsuits if it has complied with all FDA regulations. Pfizer contends that because the FDA has ultimate authority over drug labeling, state courts cannot hold the company liable for the label’s content. While a powerful defense, preemption is controversial because it can leave injured patients with no path to compensation.
Pfizer’s Argument: “The FDA Tied Our Hands”
Pfizer’s central claim is that it attempted to add a meningioma warning to Depo-Provera’s label, but the FDA rejected the change. According to Pfizer, this rejection legally prevented them from warning patients, meaning their hands were tied by federal regulators. This argument relies on the Supremacy Clause of the U.S. Constitution.
If the court accepts this defense, thousands of failure-to-warn claims could be dismissed, marking a major victory for Pfizer and a significant setback for plaintiffs.
Plaintiffs’ Rebuttal and the Latest Depo Provera Lawsuit News
Plaintiffs’ attorneys have mounted a strong counter-argument, alleging that Pfizer failed to provide the FDA with complete information about the meningioma risk. They argue that preemption only applies if a drug manufacturer has been fully transparent with regulators.
If Pfizer knew about the risk but downplayed or withheld data, the FDA’s rejection of a label change would not be a valid defense. Plaintiffs point to years of scientific evidence linking progesterone to meningioma growth and suggest Pfizer only sought a label change after pressure from European regulators. They contend Pfizer has not met the “clear evidence” standard required for a preemption defense to succeed.
The court’s decision on this motion is a critical development in the depo provera lawsuit news. A denial would allow the litigation to proceed to discovery and the key bellwether trials. A victory for Pfizer could end many cases before they truly begin.
For more insight into whether this litigation is gaining momentum, check out: Is Depo-Provera Emerging as a Major Mass Tort in 2025?.
The Science: Understanding the Link Between Depo-Provera and Brain Tumors
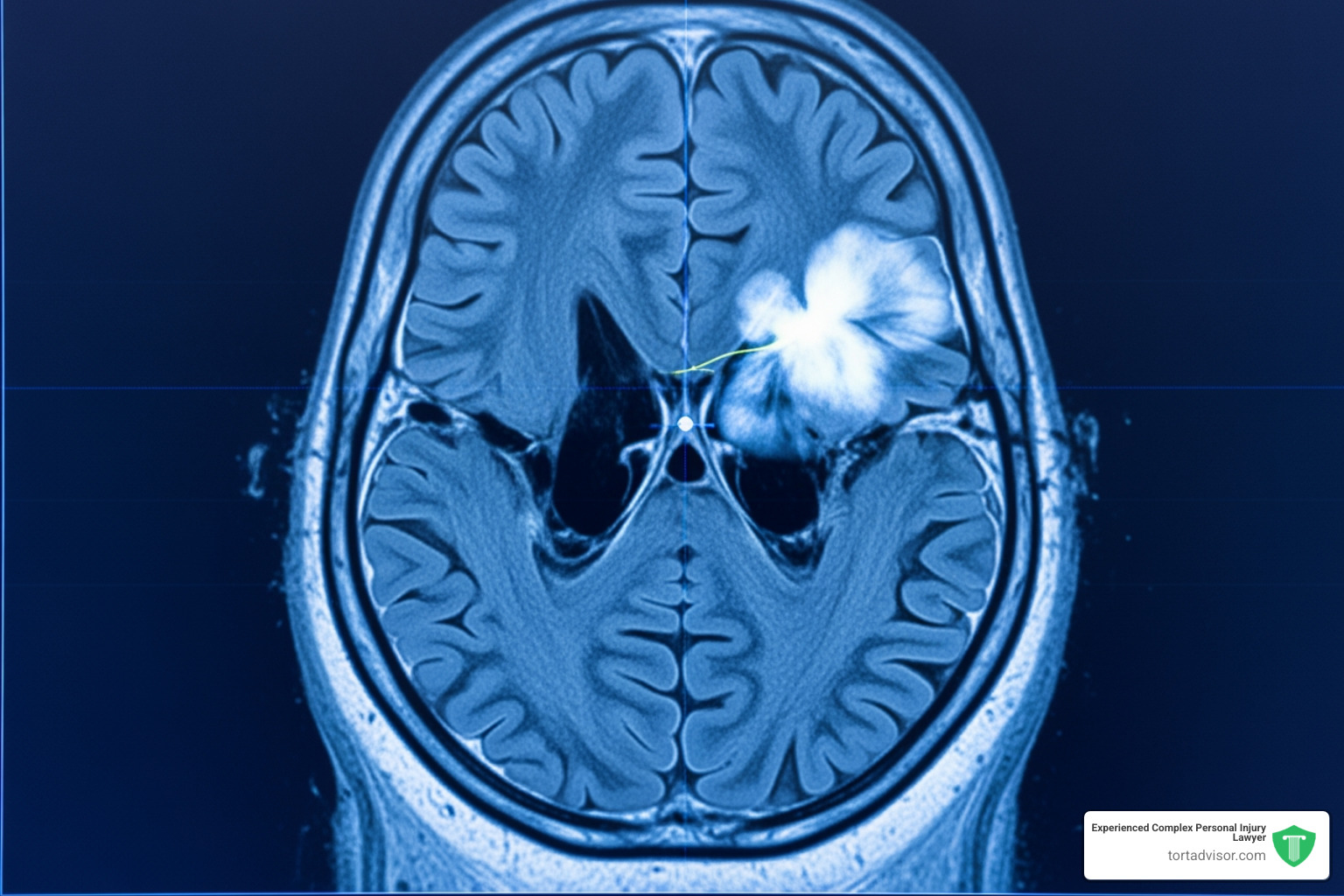
The medical condition central to these lawsuits is a meningioma, a tumor that grows in the meninges, the protective membranes covering the brain and spinal cord.
Meningiomas are the most common type of primary brain tumor. While most are technically benign (non-cancerous), they are not harmless. As a tumor grows within the skull, it presses on the brain and nerves, which can trigger severe headaches, vision loss, hearing problems, seizures, and weakness in the limbs. Some patients also experience memory issues or personality changes.
How Depo-Provera is Implicated in Meningioma Growth
The connection involves Depo-Provera’s active ingredient, medroxyprogesterone acetate (MPA), a synthetic version of the hormone progesterone. The link is that many meningiomas are hormone-sensitive tumors. Research has shown that meningioma cells often have numerous progesterone receptors on their surface.
When synthetic progestins like MPA bind to these receptors, they can stimulate tumor growth. The biological theory is that regular, high-dose injections over several years could fuel the development or growth of these hormone-sensitive tumors. This scientific foundation is a central argument in the current depo provera lawsuit news.
For a comprehensive look at the scientific evidence, visit: Does Depo-Provera Cause Brain Tumors?.
Key Evidence from a Landmark Study
A landmark study in The British Medical Journal in March 2024 significantly strengthened the scientific case against Depo-Provera. The comprehensive, peer-reviewed study found that women who used Depo-Provera for more than one year faced a 5.6-fold increased risk of developing meningiomas.
This study is not an outlier. Earlier research in journals like Cancers and Expert Opinion on Drug Safety also reported significantly lifted risks, with one study noting a 3.5-fold increased likelihood of meningiomas in long-term users compared to those on oral contraceptives. This body of research provides a robust scientific backbone for the lawsuits.
Troublingly, Canada and the European Union added warnings about the meningioma risk to Depo-Provera labels years ago. The U.S. label, however, still lacks a specific, comparable warning. This disparity is a critical point in the litigation, as plaintiffs argue American women were not given the same essential safety information as their counterparts in other countries.
For more detailed information on the connection between Depo-Provera and brain tumors, see: Depo Shot Brain Tumor.
Filing a Lawsuit: Eligibility, Compensation, and Legal Process
If you or a loved one developed a meningioma after using Depo-Provera, you may be wondering about your legal options. Here’s a breakdown of the key factors for filing a lawsuit.
Eligibility generally depends on several factors. You must have used Depo-Provera, Depo-SubQ Provera 104, or an authorized generic version for at least one year. You also need an image-confirmed diagnosis of a meningioma brain tumor from an MRI or CT scan.
Timing is also critical due to the statute of limitations, which is the legal deadline for filing. Most states allow 2-3 years from the date of diagnosis. However, the “discovery rule” may extend this period. This rule states that the filing clock doesn’t start until you discover, or reasonably should have discovered, the link between your injury and its cause. If you were diagnosed years ago but only recently learned of the connection through depo provera lawsuit news, you might still be eligible.
For a comprehensive guide on eligibility, visit: Depo-Provera Lawsuit Criteria Guide.
Potential Settlement Amounts and Compensation
While there is no global settlement yet, current estimates suggest that settlement amounts could range from $100,000 to over $1 million. The final amount depends on factors like the severity of your tumor and the extent of your damages.
Compensation typically covers:
- Medical Expenses: Costs for diagnosis, surgery, radiation, and ongoing care. Treating a meningioma can be incredibly expensive, often exceeding $700,000.
- Lost Wages: Income lost during treatment and recovery, as well as any reduction in future earning capacity due to permanent disability.
- Pain and Suffering: Compensation for the physical pain, emotional distress, and overall impact on your quality of life.
For context, past meningioma lawsuits have resulted in average settlements over $800,000 and jury verdicts exceeding $3 million. A prior Depo-Provera class action in Canada over a different injury settled for over $2 million, showing that significant compensation is possible when companies fail to warn of serious risks.
For more detailed information on potential compensation, explore: Depo-Provera Settlement Amounts Lawsuit Guide.
Your Guide to the Latest Depo Provera Lawsuit News and Legal Steps
To start, gather your medical records (MRI/CT scans, pathology reports) confirming your diagnosis and pharmacy records showing your history of Depo-Provera use. If you missed work, collect proof of lost wages.
These cases are proceeding as an MDL (Multidistrict Litigation), not a class action. Unlike a class action where a settlement is divided equally, an MDL allows each plaintiff to retain an individual case. While cases are grouped for pretrial efficiency, your potential compensation is based on your unique injuries. If your case doesn’t settle, it can still proceed to an individual trial.
An experienced pharmaceutical litigation attorney can guide you through this complex process, help gather documentation, and fight for the compensation you deserve. To connect with legal professionals experienced in this area, visit: Depo-Provera Injury Lawyer.
Frequently Asked Questions about Depo-Provera Lawsuits
If you are considering a Depo-Provera lawsuit, you likely have many questions. Here are concise answers to some of the most common ones.
What is the current status of the Depo-Provera lawsuits?
The lawsuits are actively proceeding in Multidistrict Litigation (MDL) 3140, centralized in the Northern District of Florida. As of October 2025, over 1,200 cases are part of the MDL, with more expected. Key developments include Pfizer’s motion for summary judgment based on federal preemption and the selection of five bellwether cases for initial trials. These test cases will help establish how juries might view the evidence and influence future settlement talks. There is also ongoing coordination with dozens of state court cases.
How much compensation can I get from a Depo-Provera lawsuit?
While every case is unique and there is no global settlement yet, legal experts estimate that individual settlements could range from $100,000 to over $1 million. The final amount depends on factors like the severity of your tumor, the cost of your medical treatment, your lost wages, and the impact on your quality of life. In similar mass tort cases, attorneys work on a contingency-fee basis, meaning you only pay legal fees if they successfully recover compensation for you.
Do I qualify to file a Depo-Provera lawsuit?
You may qualify if you meet these general criteria:
- You used brand-name Depo-Provera, Depo-SubQ Provera 104, or an authorized generic.
- Your use of the drug was for at least one year.
- You were subsequently diagnosed with an image-confirmed meningioma brain tumor.
- You are within your state’s statute of limitations (the legal deadline to file). This is often 2-3 years from diagnosis, but the “discovery rule” can sometimes extend this window if you only recently learned of the link between the drug and your tumor.
Because every situation is different, we strongly recommend a free, no-obligation case review with an experienced attorney to evaluate your specific circumstances and confirm your eligibility.
How to Get Help for Your Depo-Provera Injuries
The depo provera lawsuit news is developing quickly. If you were diagnosed with a meningioma after using the birth control shot, you may be entitled to significant compensation, but time is limited.
Time is a critical factor. Most states have a 2-3 year statute of limitations for filing a claim. Waiting too long could mean losing your right to seek justice. Furthermore, navigating pharmaceutical litigation requires specialized expertise. These cases involve complex science and powerful corporate legal teams, and you need skilled representation to level the playing field.
That’s where Tort Advisor comes in. We connect you with highly skilled lawyers who have proven track records in complex pharmaceutical litigation. They are specialists who understand these cases and have secured substantial compensation for their clients.
The process begins with a free, no-obligation case review. There is no upfront cost and no pressure. The attorneys we connect you with work on a contingency-fee basis, so you only pay if they win compensation for you.
After all you’ve been through, taking the first step to understand your legal options is crucial. You deserve answers and a dedicated advocate fighting for you.
To get started with a free case review and connect with an experienced attorney who can evaluate your specific situation, visit: Get a Free Case Review.
Free Confidential Case Evaluation
Complete the short form below to get an immediate FREE case review with an expert in your specific claim. Don't wait, your case could be time sensitive to file a claim.
Related Posts
Discover New Jersey disability benefits: TDI, FLI, SSDI, SSI rates, eligibility, applications & appeals for 2025-2026.
Hire a Depo-Provera lawsuit attorney now. Fight Pfizer for meningioma risks from injections. Free consult, MDL updates & settlements up to $1.5M.
Find top Miami florida car accident lawyers after your 305 crash. Get max compensation, navigate no-fault laws & choose the best experts now!
Diagnosed with cancer after Roundup? Learn about the monsanto roundup lawsuits, eligibility criteria, and how to pursue your claim.
Discover how do you qualify for a hair relaxer lawsuit: criteria, diagnoses, evidence & brands in uterine cancer MDL. Claim review now!
Find the best uber sexual assault lawsuit lawyer: expert guides, MDL experience, proven results & nationwide firms for justice.