

Understanding the Depo-Provera Bone Loss Lawsuit
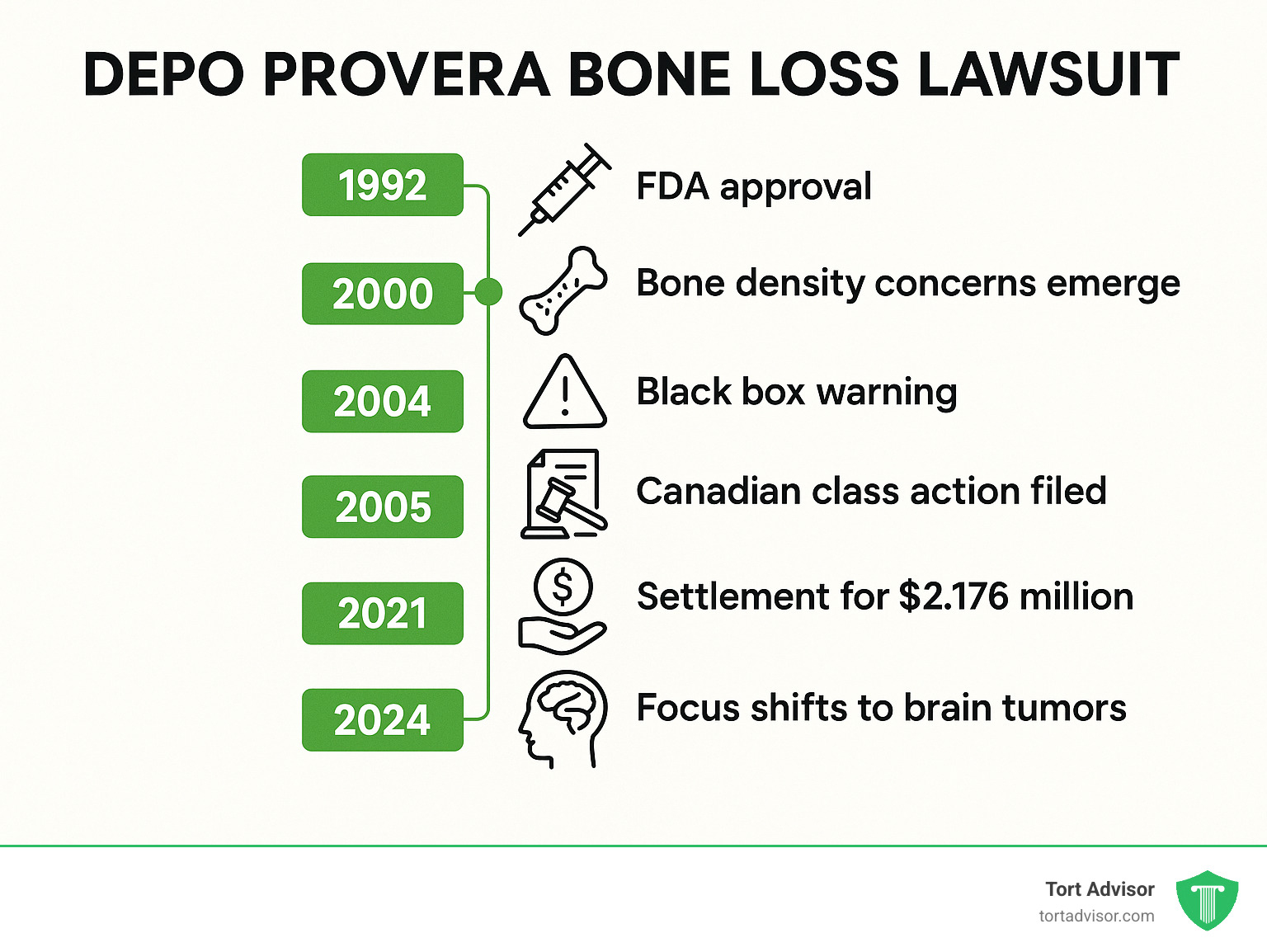
The depo provera bone loss lawsuit centers on claims that Pfizer failed to adequately warn users about the risk of significant bone density loss from their injectable contraceptive. Here are the key facts:
- Settlement Reached: In May 2021, Pfizer settled the Canadian class action for $2,176,250.
- Claims Period Ended: The deadline to file claims was March 1, 2022; this lawsuit is now terminated.
- Main Allegation: Pfizer misrepresented risks and failed to warn that Depo-Provera could cause significant, possibly irreversible bone mineral density loss.
- Current Status: While bone loss claims are largely concluded, new lawsuits now focus on brain tumors (meningiomas).
- FDA Warning: A black box warning was added in 2004 about bone density loss risks.
Depo-Provera is an injectable birth control that prevents pregnancy for three months. Research shows that users experience decreased bone mineral density, especially during the first few years. Young women appear more susceptible to this bone-thinning effect.
The legal focus has shifted significantly. While the bone density class action settled in Canada, current litigation centers on meningioma brain tumors, with cases consolidated in federal court under MDL 3140.
I’m Mason Arnao of Tort Advisor. I’ve helped connect individuals affected by pharmaceutical injuries with qualified legal representation, and I know the importance of clear, accurate information in these complex cases.
Depo provera bone loss lawsuit terms to remember:
- Depo Provera severe side effects
- birth control shot lawsuit
- depo shot brain tumor
What is Depo-Provera?
Depo-Provera is the brand name for medroxyprogesterone acetate (MPA), an injectable hormonal birth control. Approved by the FDA in 1992, its convenience made it a popular choice for long-term contraception. It prevents pregnancy by stopping ovulation, thickening cervical mucus to block sperm, and thinning the uterine lining.
However, for some users, unintended side effects led to significant health concerns and the depo provera bone loss lawsuit. For a broader look at potential harms, see our information on Depo Provera’s severe side effects.
The Emergence of Bone Loss Concerns
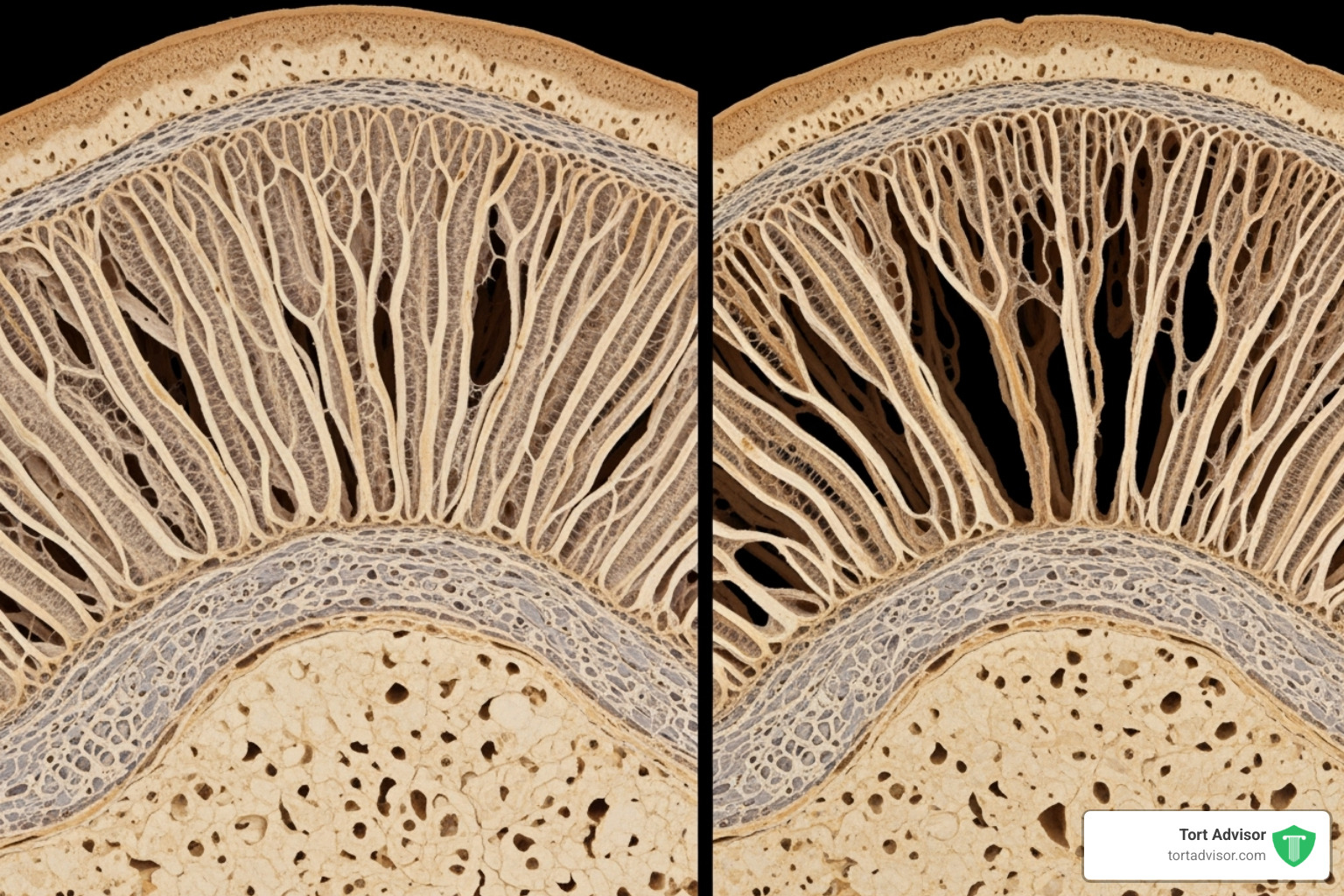
Health experts began raising concerns about Depo-Provera’s impact on bone mineral density (BMD). Investigations revealed that users could experience significant, potentially irreversible, bone loss.
This evidence led the FDA to issue a “black box” warning in 2004—its strongest alert. The warning highlighted the risk of bone density loss leading to osteopenia and osteoporosis. It also advised limiting Depo-Provera use to two years unless other birth control methods were unsuitable. This was a clear signal that the shot’s convenience came with a serious bone health risk.
The Scientific Link: How Depo-Provera Affects Bone Density
Understanding why the depo provera bone loss lawsuit emerged requires looking at the science. Research shows how Depo-Provera interferes with the body’s natural bone-building process.
Your bones constantly undergo bone remodeling, where old bone tissue is broken down by cells called osteoclasts and replaced with new tissue by cells called osteoblasts. This balanced cycle keeps bones strong.
Depo-Provera contains medroxyprogesterone acetate, which suppresses your body’s production of estrogen. Estrogen is crucial for bone health because it helps regulate the remodeling process. When Depo-Provera lowers estrogen levels, this balance is disrupted. Bone breakdown by osteoclasts can outpace bone formation by osteoblasts, causing your Bone Mineral Density (BMD) to decline and making bones more fragile.
Research has consistently shown that women using this contraceptive method experience a decrease in bone mineral density, especially in the first few years of use. This evidence was central to the legal claims.
A key question is whether this bone loss is reversible. While some women regain bone density after stopping Depo-Provera, the recovery is often incomplete. This means that even after discontinuing the shot, women may face an increased risk of osteoporosis and fractures later in life.
Symptoms of Bone Loss and Osteoporosis
Osteoporosis is often called a “silent disease” because it can progress without symptoms until a fracture occurs. For Depo-Provera users, potential signs include:
- Back pain, which can result from tiny fractures in the vertebrae.
- Loss of height over time due to compression fractures in the spine.
- Stooped posture, or a rounding of the upper back.
- Bones that break much more easily than expected from a minor fall or bump.
Who is Most at Risk?
Certain groups face higher risks of bone density loss from Depo-Provera.
- Adolescents and young women are vulnerable because they are still building their peak bone mass. Interfering with this process can have lasting consequences. Young women appear to be more susceptible to this bone-thinning effect.
- Long-term users (more than two years) face compounded risks, as bone loss increases with the duration of use.
- Women with pre-existing risk factors for osteoporosis are in the most danger. These factors include low body weight, poor nutrition (low calcium and Vitamin D), a family history of osteoporosis, smoking, and excessive alcohol use. For these women, Depo-Provera can accelerate bone loss.
Understanding these risks was crucial in legal cases arguing that Pfizer should have provided clearer warnings about who was most vulnerable.
Understanding the Depo Provera Bone Loss Lawsuit
Many women who experienced serious bone density problems after using Depo-Provera felt they were not properly warned of the risks. This led to a significant legal battle against Pfizer Inc. and Pfizer Canada ULC.
The depo provera bone loss lawsuit became a class action, representing numerous women with similar experiences. The legal fight centered on two main issues: misrepresentation and failure to warn. Plaintiffs argued that Pfizer did not provide a complete picture of the potential effects on bone health.
Main Claims in the Depo Provera Bone Loss Lawsuit
The women in the class action alleged that Pfizer:
- Failed to disclose risks: The primary complaint was that Pfizer knew about the risk of significant, irreversible bone loss but did not properly communicate it to patients and doctors.
- Misrepresented the drug’s safety: The lawsuit also claimed Pfizer portrayed Depo-Provera as safer than it was, potentially through marketing materials or information given to healthcare providers.
The lawsuit highlighted that this alleged failure to warn resulted in women developing significant and irreversible bone loss, leading to conditions like osteopenia and osteoporosis. These conditions represent increased fracture risk, chronic pain, and long-term health complications.
Outcome of the Depo Provera Bone Loss Lawsuit
After a legal battle of more than 15 years, the Canadian Class Action against Pfizer reached a resolution. In May 2021, the Superior Court of Quebec approved a settlement for $2,176,250. The settlement provided $1,913,750 to affected women and $262,500 to health insurance companies.
This lawsuit is now terminated. The claims deadline was March 1, 2022, and the window to join has closed. All approved claims were paid in 2023, and the case is officially done.
While the bone loss litigation has concluded, concerns about Depo-Provera remain. The legal focus has now shifted to a different serious concern: a potential link between Depo-Provera and meningioma brain tumors. For the women affected by bone loss, the settlement represented a measure of justice and underscored the responsibility of pharmaceutical companies to be transparent about serious risks.
Current Depo-Provera Legal Actions: A Shift in Focus
While the depo provera bone loss lawsuit has concluded, the legal landscape for Depo-Provera is not quiet. The focus has shifted to new legal actions concerning other serious alleged side effects, particularly brain tumors.
In pharmaceutical litigation, legal focus often evolves with new scientific research. For Depo-Provera, the spotlight has moved to meningioma brain tumors. This has led to a new wave of lawsuits in the United States alleging Pfizer failed to warn consumers about this risk. You can find more information on the broader Depo Provera Lawsuit and its current status.
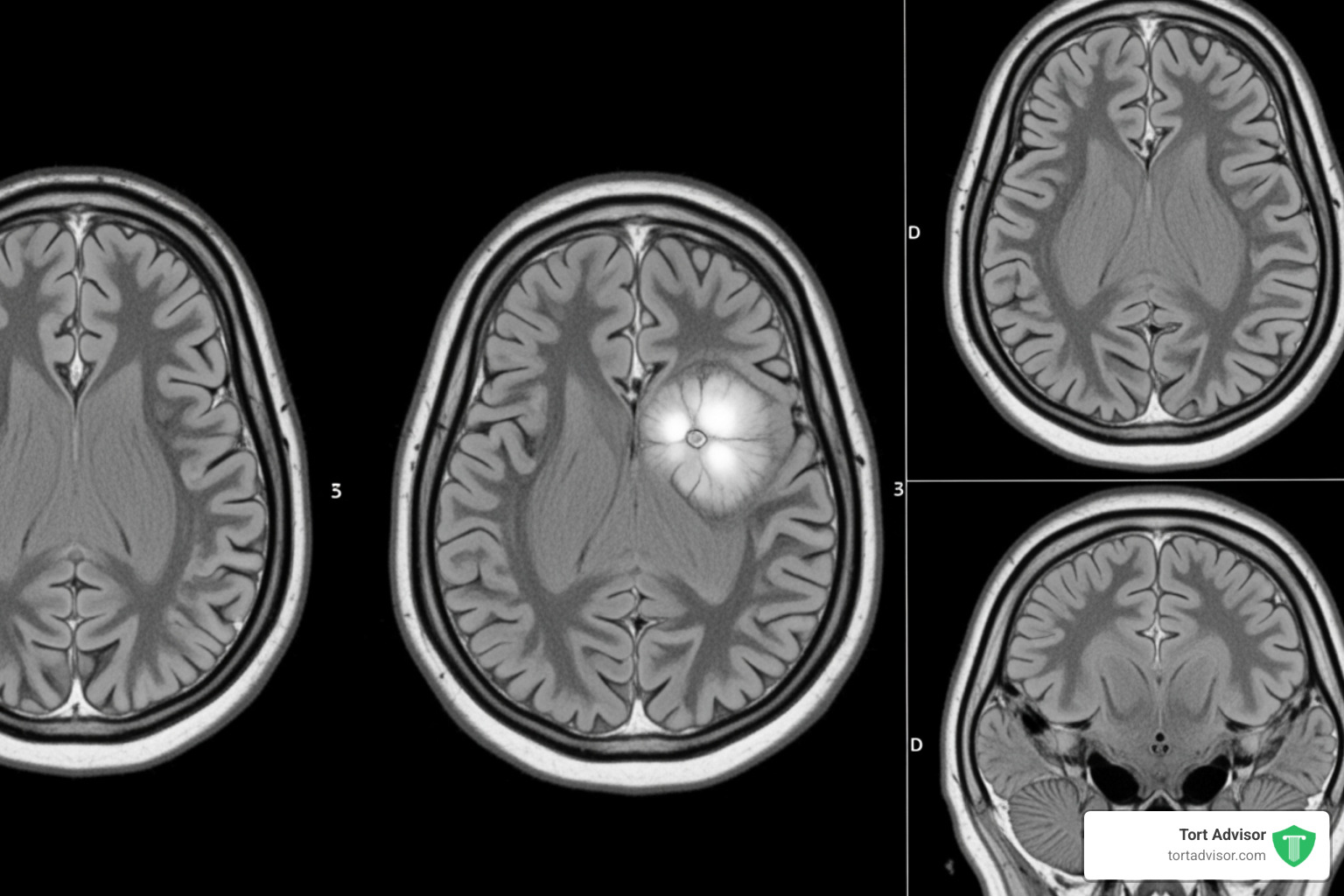
The Link to Meningioma Brain Tumors
Meningiomas are tumors in the membranes covering the brain and spinal cord. Though typically non-cancerous, they can cause serious neurological symptoms like headaches, vision loss, and seizures by pressing on the brain.
Recent scientific studies suggest a link between Depo-Provera use and an increased risk of these tumors. A study in the British Medical Journal (BMJ) found that using Depo-Provera for a year or more was associated with a five-fold higher risk of developing a meningioma requiring surgery. Other research suggests a 5.6 times greater risk for long-term users.
The biological reason may be that meningioma cells often have progesterone receptors. Since Depo-Provera is a synthetic progestin, it could stimulate tumor growth. This science is the foundation of ongoing litigation alleging Pfizer failed to warn users.
Are There Other Ongoing Lawsuits?
Yes, but the current litigation for meningioma brain tumors in the U.S. is not a class action. Instead, individual lawsuits are consolidated into a Multidistrict Litigation (MDL No. 3140, Depo-Provera Products Liability Litigation).
An MDL centralizes similar individual lawsuits into one court for streamlined pretrial proceedings, like findy. However, each plaintiff’s case remains individual. As of late 2024 and early 2025, the focus of new lawsuits is almost exclusively on meningioma. While the link to bone loss is well-established, the legal avenues for those claims have largely closed. For more on the current legal landscape, see our page on the Depo Provera Class Action Lawsuit 2024, which explains the shift to MDLs.
What to Do If You’ve Been Affected by Depo-Provera
If you used Depo-Provera and now have health concerns, you can take steps to protect your health and legal rights. The first step is a medical consultation. Be honest with your provider about your complete Depo-Provera history and any symptoms.
For bone health concerns, your doctor may recommend a bone density scan (DEXA scan). This test measures bone mineral density and can diagnose osteopenia or osteoporosis.
It is also important to document your medical history. Gather all relevant records, including Depo-Provera prescriptions, medical visits, test results, and treatments. A detailed prescription history is especially valuable. The more complete your timeline, the stronger your potential case.
Finally, consider speaking with a legal professional specializing in pharmaceutical cases. An experienced attorney can help you understand your legal options, as the depo provera bone loss lawsuit has concluded, but new litigation focuses on brain tumors.
Steps to Protect Your Health
Taking control of your health is about protecting your future. Here are steps you can take to support your bone health:
- Consult your doctor: Discuss your concerns, bone density results, and long-term health plan. If you are still using Depo-Provera, discuss alternative contraceptives.
- Make lifestyle changes: Your bones respond to how you treat them. Ensure adequate Calcium and Vitamin D intake through diet (dairy, leafy greens) or supplements.
- Engage in weight-bearing exercise: Activities like walking, dancing, or climbing stairs signal your body to build bone tissue. Consistency is key.
Seeking Legal Guidance
If you consider legal action, timing is critical. The statute of limitations varies by state and can be short. Acting promptly is essential to preserve your rights.
- Collect evidence: Gather all medical records and bills to create a timeline of your health journey and its financial impact.
- Document symptoms and life impact: Keep a journal detailing how your symptoms affect daily activities, work, and your quality of life.
While the major depo provera bone loss lawsuit is over, an experienced attorney can evaluate your specific circumstances, especially regarding brain tumors, and determine the best path forward.
Frequently Asked Questions about Depo-Provera and Bone Loss
Here are answers to common questions about Depo-Provera, bone loss, and your legal options.
Is the bone loss from Depo-Provera permanent?
This is a key question behind the depo provera bone loss lawsuit. The answer is complex.
Bone density generally improves after stopping the drug as the body attempts to heal. However, the recovery may not return you to baseline. Research shows many women do not fully regain their prior bone density, especially after more than two years of use. The FDA’s black box warning states the bone loss may be “possibly irreversible.”
This means you could face an increased fracture risk during and after use. The extent of recovery varies by individual and is influenced by age, duration of use, genetics, and lifestyle.
Can I still file a Depo-Provera bone loss lawsuit?
Unfortunately, no. The major Canadian class action lawsuit regarding bone loss has concluded.
That depo provera bone loss lawsuit settled in May 2021, and the deadline for claims was March 2022. The case is now officially closed and terminated. It is not possible to join it now.
Current legal focus has shifted to brain tumors (meningiomas). While bone loss is a serious concern, the primary legal avenue for those claims has closed. If you are dealing with meningioma issues from Depo-Provera use, legal options may still be available.
What is the FDA’s current warning on Depo-Provera?
In 2004, the FDA added a “black box” warning to Depo-Provera’s label—its most serious alert.
This warning advises that prolonged use may result in significant and possibly irreversible bone density loss. It is a clear statement to doctors and patients about this serious risk.
The FDA also recommends against use for longer than two years unless other methods are inadequate. This suggests that after two years, the bone loss risk becomes so concerning that continued use should only be considered if there are no other viable birth control options.
This warning remains in place today, highlighting why the depo provera bone loss lawsuit occurred: the risks were significant and often inadequately communicated to users.
How to Get Help for Your Depo-Provera Injuries
If you used Depo-Provera and later developed serious health problems like osteoporosis or a meningioma brain tumor, understanding your legal options is crucial. While the depo provera bone loss lawsuit has concluded, you may still have rights, especially if you’re dealing with other Depo-Provera-related injuries.
Navigating the legal system while managing health concerns is overwhelming. The right support is essential.
Tort Advisor specializes in connecting individuals with experienced attorneys who understand pharmaceutical injury cases. We partner with highly skilled attorneys who have proven track records in cases involving drug manufacturers.
Time is a critical factor. Statutes of limitations vary by state, and waiting too long could mean losing your right to seek compensation. This is especially true for emerging claims related to meningioma brain tumors.
Our partner attorneys can evaluate your specific situation to determine if you have a viable case. They will review your medical history, Depo-Provera use, and the severity of your injuries to fight for the compensation you deserve.
Most pharmaceutical injury cases are handled on a contingency fee basis, so you pay no attorney fees unless you win your case. This removes the financial barrier to seeking justice.
To understand your legal options and take the first step, explore our comprehensive Depo Provera Lawsuit information page. Let us connect you with an attorney who can fight for the justice you deserve.
Free Confidential Case Evaluation
Complete the short form below to get an immediate FREE case review with an expert in your specific claim. Don't wait, your case could be time sensitive to file a claim.
Related Posts
Discover New Jersey disability benefits: TDI, FLI, SSDI, SSI rates, eligibility, applications & appeals for 2025-2026.
Hire a Depo-Provera lawsuit attorney now. Fight Pfizer for meningioma risks from injections. Free consult, MDL updates & settlements up to $1.5M.
Find top Miami florida car accident lawyers after your 305 crash. Get max compensation, navigate no-fault laws & choose the best experts now!
Diagnosed with cancer after Roundup? Learn about the monsanto roundup lawsuits, eligibility criteria, and how to pursue your claim.
Discover how do you qualify for a hair relaxer lawsuit: criteria, diagnoses, evidence & brands in uterine cancer MDL. Claim review now!
Find the best uber sexual assault lawsuit lawyer: expert guides, MDL experience, proven results & nationwide firms for justice.