Understanding the 2023 Breast Implant Recall Updates
The breast implant recall 2023 continues to impact thousands of patients who received textured breast implants, particularly those manufactured by Allergan. While the major recall began in 2019, new safety data and cancer reports emerged throughout 2023 that changed how doctors and patients view implant risks.
Key 2023 Updates:
– 19 new cases of squamous cell carcinoma (SCC) reported around breast implants
– 1,264 total cases of breast implant-associated lymphoma (BIA-ALCL) worldwide
– 63 deaths linked to BIA-ALCL as of June 2023
– New FDA safety communication issued in March 2023 about additional cancer risks
The recall primarily affects textured implants – specifically Allergan’s Biocell line including Natrelle saline, Natrelle silicone, Natrelle Inspira, and Natrelle 410 models. These textured surfaces were linked to significantly higher rates of a rare lymphoma called BIA-ALCL.
Most important fact: The FDA does not recommend automatic removal of recalled implants if you have no symptoms. However, patients should monitor for warning signs like swelling, lumps, or fluid buildup around the implant site.
Research published in 2023 showed the risk of developing BIA-ALCL is 1 in 2,200 patients with certain textured implants – much higher than previously estimated. The cancer typically develops 8 years after implantation and has a 93% survival rate when caught early.
Quick Facts & Timeline of Recalls 2019-2025
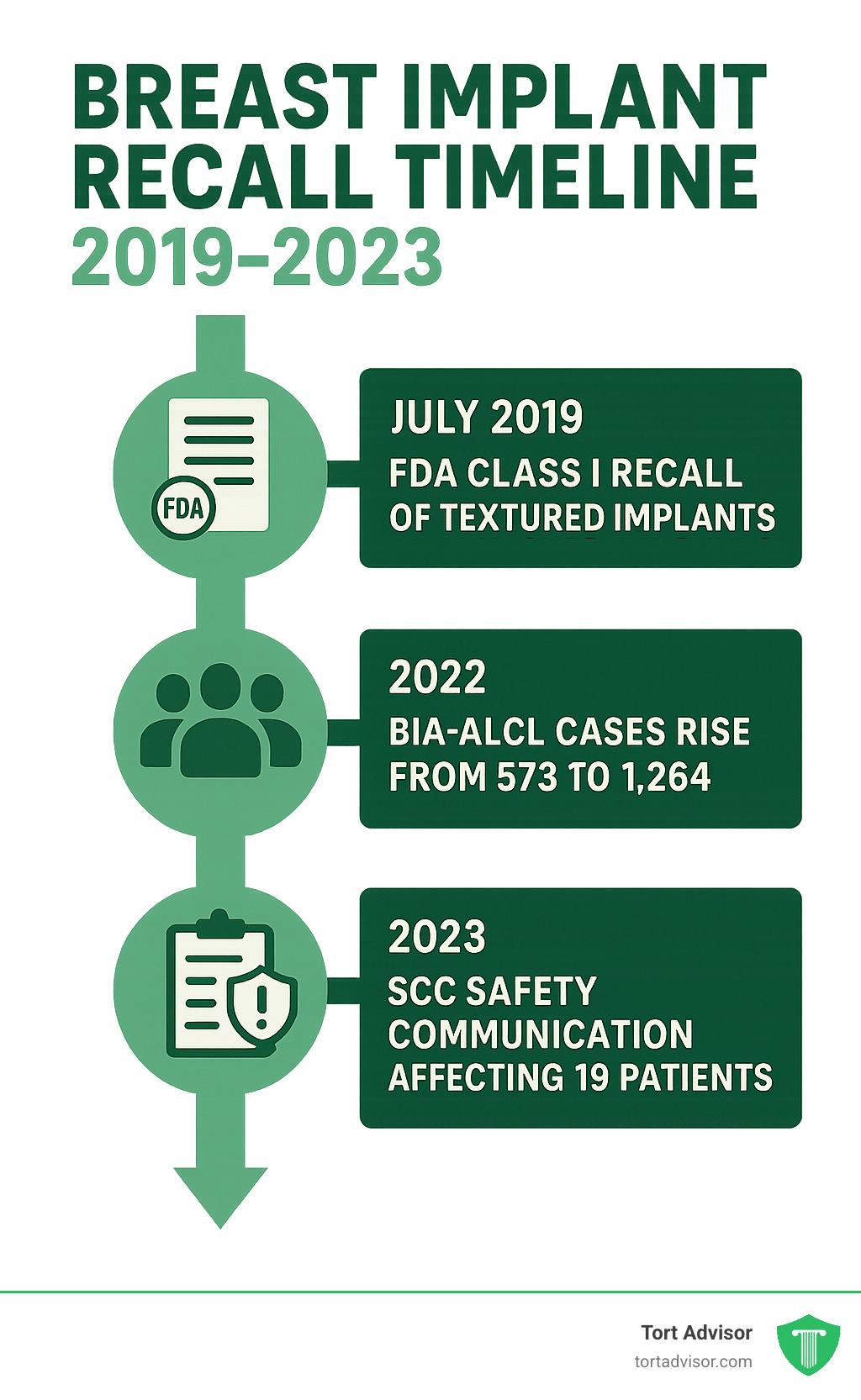
The breast implant recall 2023 story began in July 2019 when the FDA asked Allergan to voluntarily recall their Biocell textured breast implants. This Class I recall affected over 4 million implants globally after 481 of 573 BIA-ALCL cases worldwide involved Allergan products.
What followed was a cascade of legal and medical developments. In 2020, federal courts created MDL 2921 to handle thousands of similar cases. The FDA issued a warning letter to Allergan for failing to complete required safety studies.
Major Milestones Since 2019
The most significant development came in March 2023 when the FDA issued a new safety communication about squamous cell carcinoma (SCC) cases around breast implants. This expanded understanding of implant risks beyond BIA-ALCL.
Cancer numbers climbed from 573 BIA-ALCL cases in 2019 to 1,264 cases with 63 deaths by 2023. The FDA’s database showed 1,130 BIA-ALCL reports by August 2022, with 953 linked to Allergan implants.
Snapshot of Devices Affected
The recall targeted specific Allergan products: Natrelle saline and Natrelle silicone implants, Natrelle Inspira models, teardrop-shaped Natrelle 410 implants, and 133 Plus tissue expanders.
The key difference is surface texture. Textured implants have rough outer shells designed to grip tissue, while smooth implants have slippery surfaces that appear much safer from a cancer standpoint. While smooth implants have been associated with only a handful of BIA-ALCL cases worldwide, certain textured models showed unacceptable cancer rates.
What’s New in the Breast Implant Recall 2023?
The breast implant recall 2023 updates brought serious news that changed everything about implant safety understanding. The biggest development came in March 2023 when the FDA issued a safety communication about squamous cell carcinoma (SCC) – a completely different cancer from BIA-ALCL.
Unlike BIA-ALCL which happens in fluid around implants, SCC grows in scar tissue that naturally forms around any implant. This means even patients with smooth implants could be at risk.
The 2023 numbers were significant: 19 SCC cases reported affecting 17 women, 1 man, and 1 unspecified person. These cancers developed 7 to 42 years after implant surgery. BIA-ALCL cases had climbed to 1,264 worldwide with 63 deaths.
Fresh Research & Statistics
New research provided precise risk numbers: 30.54 cases per 100,000 patients with textured implants, or about 1 case for every 3,274 patients – much higher than expected.
For all implant types together: 6.70 cases per 100,000 patients, or about 1 case for every 14,925 patients.
Key patterns emerged: Women over 46 were more at risk, especially with implants for more than 6 years. Macro-textured implants were involved in 73% of BIA-ALCL cases. The average time between implantation and cancer was 8 years, ranging from 2 to 32 years.
Other Cancers Under Watch
SCC was particularly concerning because it could happen with any type of implant and took much longer to develop – up to 42 years after surgery. The FDA expanded reporting requirements and created the PROFILE registry to track these cases.
The Scientific research on SCC cases showed how much we still don’t know about long-term implant safety.
Understanding the Health Risks & Symptoms
Recalled breast implants present several health risks beyond cancer. While the breast implant recall 2023 focused on cancer risks, patients can experience mechanical problems, immune responses, or “Breast Implant Illness” symptoms.
Main health concerns include: BIA-ALCL, squamous cell carcinoma, Breast Implant Illness symptoms, capsular contracture, and mechanical failures like rupture.
BIA-ALCL Warning Signs
BIA-ALCL usually appears as “late seroma” – fluid collecting around your implant years after surgery. It typically shows up around 8 years after implantation, though it can occur 2 to 28 years later.
Watch for these warning signs: new swelling developing months or years after surgery, persistent fluid collection, lumps in breast or under arm, breast asymmetry developing over time, pain around the implant area, and skin changes on the breast.
Most cases affect one breast initially. When caught early and treated with complete implant and capsule removal, BIA-ALCL has a 93% disease-free survival rate at three years.
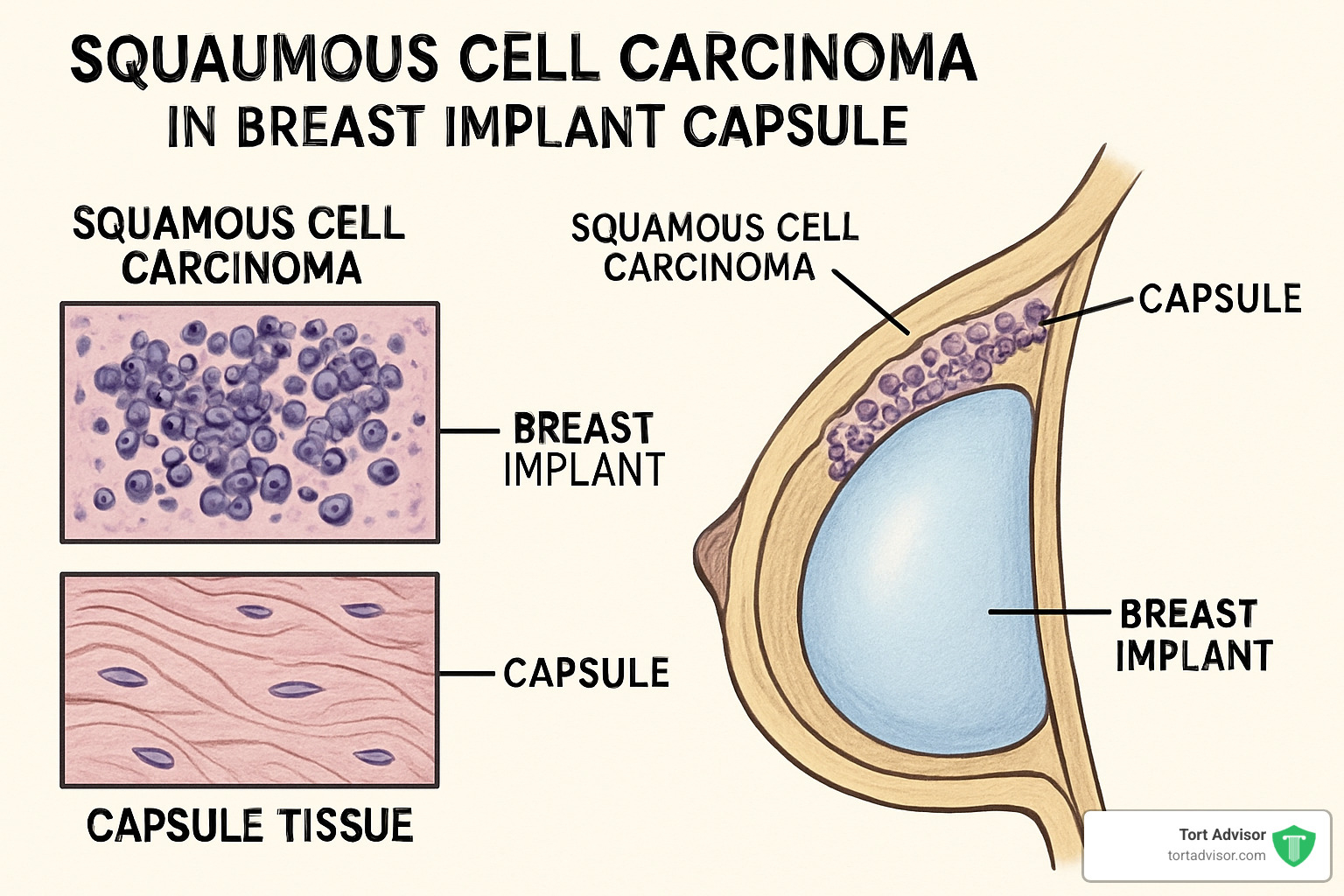
SCC & Rare Tumors Around Implants
SCC develops in capsule tissue and can take much longer to appear – 7 to 42 years after implantation. Unlike BIA-ALCL, SCC has been reported with both textured and smooth implants.
SCC symptoms include: persistent pain around the implant, skin ulceration, hard lumps in breast tissue, changes in breast shape or size, and skin discoloration or thickening.
The Scientific research on SCC cases helps doctors recognize these rare cancers. Any persistent, unexplained symptoms around implants deserve medical attention, especially if you’ve had them for many years.
What to Do if Your Implant Is on the Recall List
Finding out your implants are recalled can feel overwhelming, but remember: the FDA doesn’t recommend rushing to remove recalled implants if you have no symptoms.
First, check your implant card with manufacturer name, model number, and serial numbers. If you’ve lost it, contact your surgeon’s office for your operative report and implant details.
Next steps: Contact your surgeon’s office for complete records, verify your implants against FDA recall lists, schedule an appointment to discuss monitoring options, and report any symptoms through FDA’s MedWatch system.
Should Asymptomatic Patients Remove Recalled Implants?
The FDA’s stance is clear: don’t remove recalled implants if you have no symptoms. However, individual factors matter: age over 46 increases risk, as does having implants for more than six years.
Some patients choose removal for peace of mind, while others prefer monitoring with regular check-ups. Options include removal without replacement, exchange for smooth implants, or varying degrees of capsule removal.
Step-by-Step: Verifying & Reporting
Gather information: Request complete surgical records from your surgeon’s office. Check official sources: Use FDA databases and manufacturer websites to verify recall status. Schedule consultation: Discuss your situation with healthcare providers. Document everything: Keep copies of records and note any changes. Report problems: Use FDA’s MedWatch system for any symptoms.
If you’ve experienced complications, consider consulting with attorneys specializing in medical device cases. At Tort Advisor, we connect patients with top-rated attorneys experienced in breast implant litigation. For detailed information, check out More info about Breast Implant Lawsuits.
Legal & Financial Options Heading Into 2025
Understanding legal and financial options is crucial as we move into 2025. Most breast implant lawsuits against Allergan are consolidated in MDL 2921 in New Jersey federal court, with over 1,200 active cases remaining.
Allergan’s warranty program offers replacement implants and limited coverage, but doesn’t address pain and suffering, lost wages, or emotional trauma. The Women’s Health and Cancer Rights Act requires most insurance plans to cover breast reconstruction complications.
How Claims & Settlements Work
Litigation centers on failure to warn about cancer risks, design defects in textured surfaces, and negligent post-market surveillance. Evidence needed includes medical records, pathology reports, imaging studies, and treatment documentation.
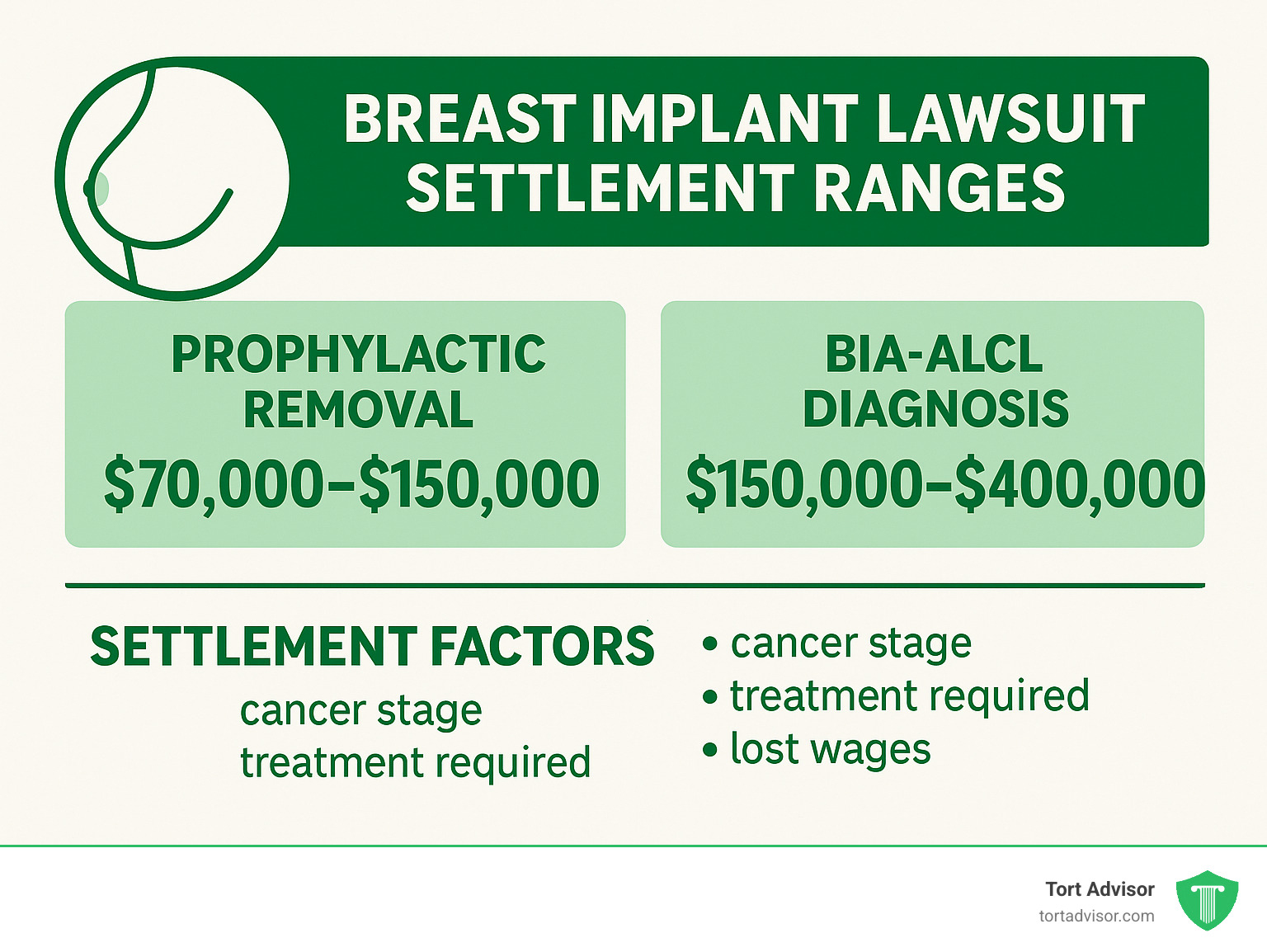
Settlement amounts vary: BIA-ALCL patients generally see $150,000 to $400,000, while prophylactic removal cases might receive $70,000 to $150,000. Statute of limitations rules vary by state – don’t assume you’ve missed your window.
Alternatives After Explantation
Smooth implants remain popular with excellent safety profiles. Fat transfer offers natural results using your own tissue. Going flat has gained acceptance with strong support communities. Combination approaches blend techniques for optimal results.
Work with board-certified plastic surgeons experienced in post-removal reconstruction. At Tort Advisor, we connect patients with experienced attorneys specializing in medical device litigation across all 50 states. Visit our Breast Implant Class Action Settlement page for detailed information.
Frequently Asked Questions about breast implant recall 2023
What brands and models are included in the 2023 update?
The breast implant recall 2023 refers to new safety information about the original 2019 Allergan recall, not new devices being recalled. We’re still talking about Allergan’s Biocell textured implants – the Natrelle saline and silicone models, Natrelle Inspira line, Natrelle 410 implants, and tissue expanders.
What changed in 2023 was understanding that cancer risks aren’t limited to recalled textured implants. The 19 squamous cell carcinoma cases included patients with both textured and smooth implants from various manufacturers.
Do I need MRI or ultrasound if I have no symptoms?
The FDA doesn’t require monitoring, but most doctors recommend it for recalled implants. Ultrasound is usually first choice – less expensive and great at spotting fluid. Many doctors suggest yearly ultrasounds for recalled implants, especially if you’ve had them over six years.
MRI is the gold standard for detecting silent ruptures but costs more. Most experts recommend MRI every 2-3 years or if ultrasound shows concerns. Your age and risk factors influence monitoring frequency.
Will Allergan or insurance pay for my removal surgery?
Allergan’s warranty program provides free replacement implants and limited diagnostic coverage, but surgery cost coverage is limited and inadequate for many patients. It doesn’t cover pain and suffering, lost wages, or other damages.
Insurance coverage varies. Cancer or ruptured implants usually qualify as medically necessary. Prophylactic removal is harder to get covered. The Women’s Health and Cancer Rights Act helps reconstruction patients fight for coverage.
Expect out-of-pocket costs between $5,000 and $15,000. Don’t assume others will pay – plan to cover costs yourself, then be surprised if warranty or insurance helps more than expected.
Conclusion
The breast implant recall 2023 continues as an ongoing patient safety story affecting thousands worldwide. New cancer data throughout 2023 fundamentally changed our understanding of textured breast implant risks.
What started as concern about BIA-ALCL has expanded to include squamous cell carcinoma and other risks. The numbers are sobering: 1,264 total BIA-ALCL cases with 63 deaths, plus 19 new SCC cases in 2023. Yet millions have textured implants, and most will never develop cancer.
Key takeaway: This recall affects specific Allergan textured implants, not all breast implants. The FDA’s position remains clear – don’t rush to remove asymptomatic recalled implants, but stay vigilant about monitoring.
Your action plan: Verify if you have recalled implants, establish monitoring with your healthcare provider, learn warning signs of BIA-ALCL and SCC, and keep detailed records. Don’t let anxiety overwhelm you – focus on facts and work with qualified professionals.
Legal options remain available for patients harmed by recalled implants. Multidistrict litigation continues, and new cases are still filed. If you’ve been diagnosed with BIA-ALCL, SCC, or other serious complications, you may be entitled to compensation.
At Tort Advisor, we connect patients with highly skilled attorneys who have proven track records in medical device litigation. Our network includes top-rated specialty attorneys in all 50 states who understand breast implant case complexities.
Whether dealing with cancer diagnosis, considering prophylactic removal, or wanting to understand legal rights, we can connect you with the right representation. For more information, visit our More info about personal-injury lawyers page.
Looking ahead to 2025, expect continued developments in safety research, ongoing litigation, and potentially new FDA guidance. Stay informed while not letting fear drive decisions. Work with qualified providers, keep detailed records, and remember you have the right to make informed choices about your health and legal options.
You don’t have to steer this alone. Resources are available to help you make the best decisions for your unique situation.
Free Confidential Case Evaluation
Complete the short form below to get an immediate FREE case review with an expert in your specific claim. Don't wait, your case could be time sensitive to file a claim.
Related Posts
Discover New Jersey disability benefits: TDI, FLI, SSDI, SSI rates, eligibility, applications & appeals for 2025-2026.
Hire a Depo-Provera lawsuit attorney now. Fight Pfizer for meningioma risks from injections. Free consult, MDL updates & settlements up to $1.5M.
Find top Miami florida car accident lawyers after your 305 crash. Get max compensation, navigate no-fault laws & choose the best experts now!
Diagnosed with cancer after Roundup? Learn about the monsanto roundup lawsuits, eligibility criteria, and how to pursue your claim.
Discover how do you qualify for a hair relaxer lawsuit: criteria, diagnoses, evidence & brands in uterine cancer MDL. Claim review now!
Find the best uber sexual assault lawsuit lawyer: expert guides, MDL experience, proven results & nationwide firms for justice.