The Truth About Breast Implant Lawsuit Silicone Toxicity
If you’re researching breast implant lawsuit silicone toxicity, here’s what you need to know:
- Litigation Status: Major class action settlements occurred in the 1990s ($3.4 billion), with newer litigation focused on BIA-ALCL cancer linked to textured implants
- Scientific Evidence: Multiple large epidemiological studies found no confirmed link between silicone implants and systemic diseases
- Common Claims: Failure to warn, design defect, negligence, and breach of warranty
- Local Complications: Capsular contracture, rupture, scarring, and inflammation are well-documented
- Current FDA Position: Silicone implants are approved but require regular monitoring for complications
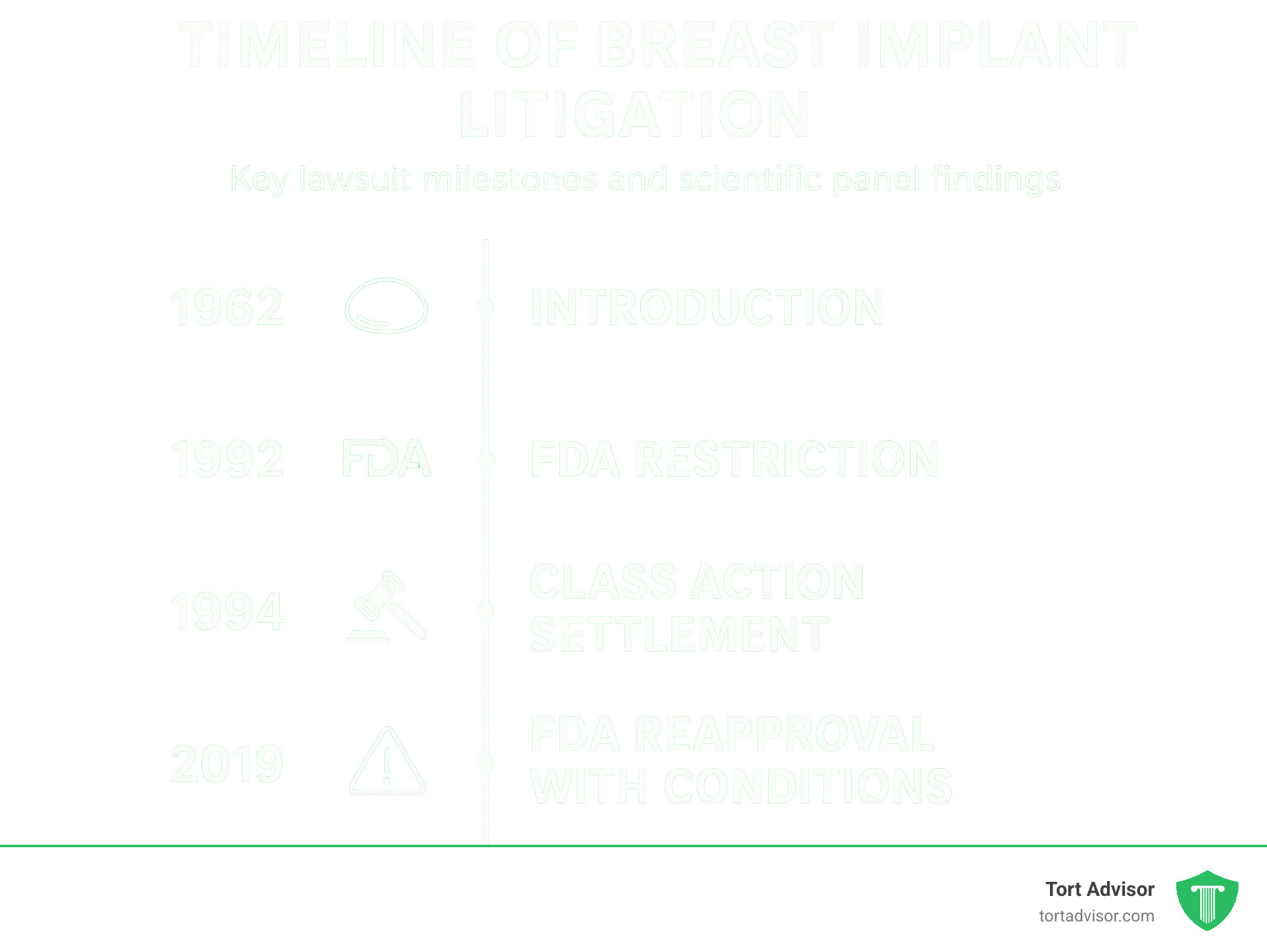
The journey through breast implant lawsuit silicone toxicity litigation has been a rollercoaster of scientific controversy, legal battles, and patient advocacy. Since their introduction in 1962, silicone breast implants have been at the center of one of the most significant mass tort litigations in American history. What began as individual cases in the 1980s exploded into hundreds of thousands of lawsuits by the 1990s, with women claiming their implants caused debilitating autoimmune diseases and systemic toxicity.
Despite the FDA’s 1992 restriction of silicone implants and billions in settlements, the scientific community remains divided. While major scientific panels found insufficient evidence linking implants to systemic diseases, many women continue to report clusters of symptoms collectively known as “breast implant illness.”
Today’s litigation landscape has shifted toward specific complications like breast implant-associated anaplastic large cell lymphoma (BIA-ALCL), with over 700 cases reported worldwide, predominantly involving textured implants.
My name is Mason Arnao, and as a legal researcher who has tracked breast implant lawsuit silicone toxicity cases for over a decade, I’ve witnessed how the intersection of science, law, and public opinion has shaped outcomes for thousands of claimants. Let’s explore what you need to know if you believe you’ve been harmed by silicone breast implants.
Decoding Silicone Toxicity: Symptoms & Science
When we talk about breast implant lawsuit silicone toxicity, we’re referring to potential health effects that can occur when silicone from breast implants interacts with the body. Silicone is a synthetic polymer made from silicon, oxygen, carbon, and hydrogen – and despite being considered relatively inert by manufacturers, evidence suggests it may not be as harmless as once thought.
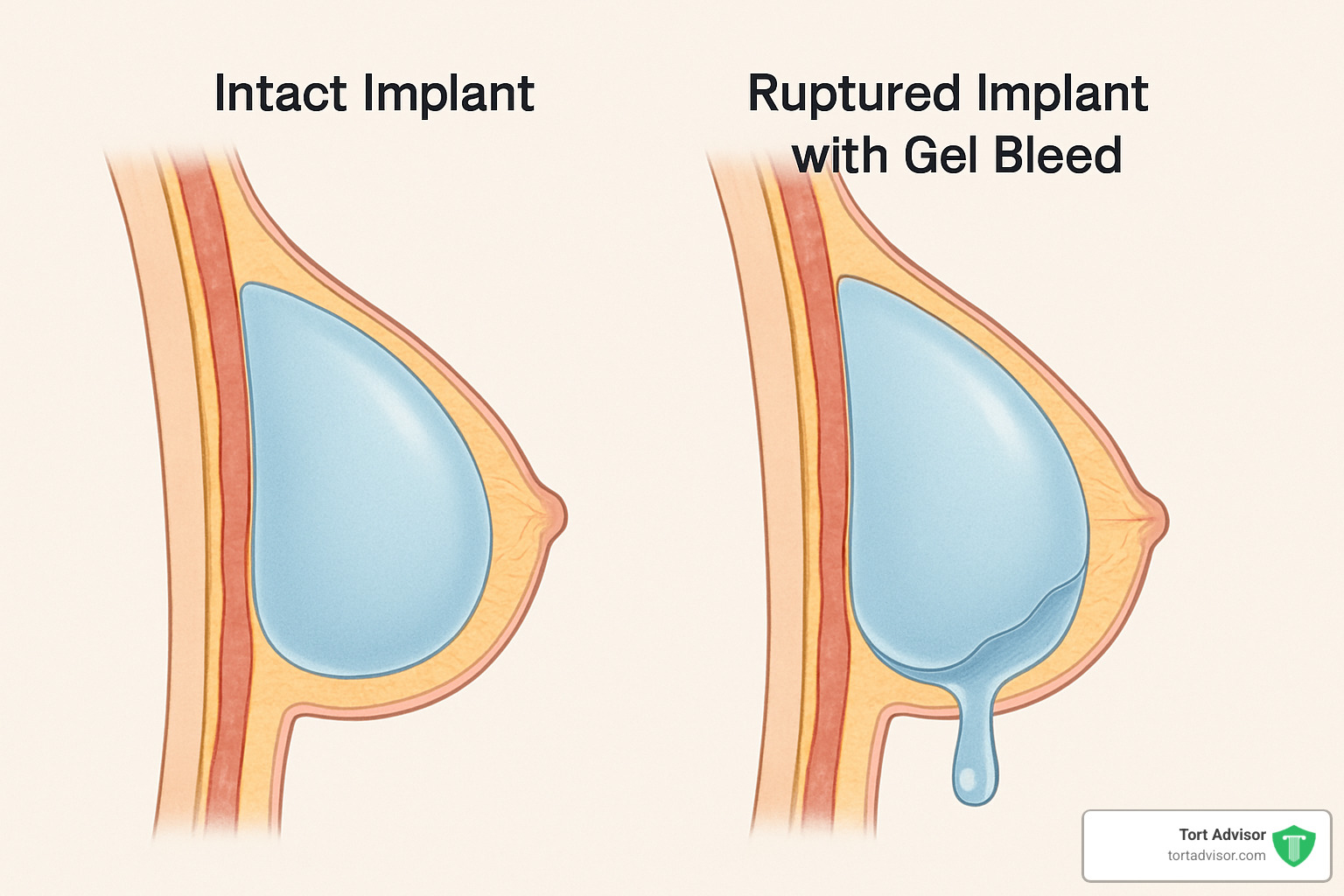
The primary concern involves “gel bleed,” where small molecules of silicone can migrate through the implant shell even without rupture. Low molecular weight silicones may leak into surrounding tissues and potentially enter the bloodstream or lymphatic system.
Reported symptoms typically fall into two categories:
- Local complications: Capsular contracture (hardening of scar tissue around implants), inflammation, pain, and cosmetic deformities
- Systemic effects: Fatigue, joint pain, cognitive issues, rashes, and autoimmune-like symptoms
One of the most serious complications is breast implant-associated anaplastic large cell lymphoma (BIA-ALCL), a rare type of non-Hodgkin’s lymphoma. According to FDA data, over 700 cases have been reported worldwide, with 36 deaths attributed to this condition.
Siliconosis & “Breast Implant Illness”
“Siliconosis” describes a constellation of symptoms reported by some women with silicone breast implants. These symptoms often mimic autoimmune disorders but may not fit neatly into established medical diagnoses.
Common symptoms include:
- Fatigue and brain fog: Persistent exhaustion and difficulty concentrating
- Joint pain and muscle aches: Widespread pain similar to fibromyalgia
- Skin rashes and sensitivity: Unexplained dermatological reactions
- Chest pain or tightness: Discomfort in the breast or chest area
- Hair loss: Thinning hair or unusual shedding
- Respiratory issues: Shortness of breath or chronic cough
In severe cases, silicone migration can lead to more serious complications. One documented case involved a woman who developed pulmonary fibrosis and embolism secondary to silicone implant leakage.
While many physicians remain skeptical about systemic effects, patient advocacy groups point to thousands of women who report symptom improvement after implant removal (explantation).
Laboratory Evidence of Harm
Recent scientific research has begun to shed light on potential mechanisms of silicone toxicity at the cellular level. A 2020 study published in Scientific Reports demonstrated that low molecular weight silicones can induce cell death in laboratory settings.
The research found that:
- Cyclic silicone compounds (particularly D4) can trigger apoptosis (programmed cell death) in human cells
- The toxicity is size-dependent, with smaller molecules being more harmful
- Cell death occurs through activation of the intrinsic apoptotic pathway
- Different cell types show varying sensitivity to silicone exposure
This laboratory evidence provides a potential biological mechanism for the symptoms reported by some implant recipients.
Major Panel Conclusions
Over the decades, several major scientific panels have evaluated the evidence regarding silicone breast implant safety:
Institute of Medicine (1999): After reviewing over 1,200 papers and 17 epidemiological studies, the IOM concluded there was no convincing evidence linking silicone breast implants to systemic diseases. However, they acknowledged frequent local complications.
American College of Rheumatology (1995): The ACR issued a statement asserting that evidence was “compelling” that silicone implants expose patients to no demonstrable risk for connective-tissue or rheumatic disease.
FDA Updates (2020): While maintaining approval for silicone implants, the FDA has increased monitoring requirements and added warnings about BIA-ALCL. The agency now recommends MRI screening for silent rupture 5-6 years after implantation and every 2-3 years thereafter.
Understanding Breast Implant Lawsuit Silicone Toxicity
When we talk about breast implant lawsuit silicone toxicity, we’re discussing a complex intersection of medical complications, patient experiences, and scientific evidence that form the foundation of these legal claims.
Let’s start with capsular contracture – a complication that affects up to 20% of women with implants. This occurs when the body forms unusually hard scar tissue around the implant, often leading to pain, discomfort, and visible breast deformity.
Then there’s the alarming reality of rupture rates. Studies have revealed that about 40% of implants fail within just six years, and by the 12-year mark, that number jumps to a staggering 63%. That’s more than half of all implants failing over time.
Perhaps most concerning are “silent leaks” – a problem unique to silicone implants. Unlike saline implants that visibly deflate when they rupture, silicone implants can leak without showing any immediate symptoms. The gel often stays trapped within the capsule of scar tissue, making these ruptures difficult to detect without specialized imaging like MRIs.
It’s worth noting that the FDA classifies silicone breast implants as Class III medical devices – their highest risk category requiring premarket approval.
What Plaintiffs Allege About Silicone Toxicity
Women filing breast implant lawsuit silicone toxicity cases typically make several key allegations against manufacturers. First, they often claim negligent design – arguing that companies knew or should have known about fundamental flaws that allow silicone to leak or rupture.
Failure to warn is another common claim. Plaintiffs argue that manufacturers didn’t adequately disclose known risks to patients or their physicians, leaving women unable to make truly informed decisions about their bodies.
Some cases include allegations of fraudulent concealment – that manufacturers deliberately hid internal research showing potential harms from regulators and the public.
Many lawsuits also include claims of breach of warranty, arguing that the implants simply didn’t perform as guaranteed by the manufacturers.
The landmark case that opened the floodgates was Maria Stern’s 1984 lawsuit against Dow Corning. During this case, internal company memos emerged suggesting manufacturers may have known about potential risks while continuing to market implants as safe.
Key Scientific Panels & FDA Findings
The evolution of breast implant lawsuit silicone toxicity litigation has been heavily shaped by scientific panel findings over the years.
The Institute of Medicine’s 1999 report marked a significant turning point. After extensive review, they concluded that diseases or conditions such as connective tissue diseases, cancer, neurological diseases or other systemic complaints are no more common in women with breast implants than in women without implants.
Multiple studies published in the New England Journal of Medicine between 1992 and 1995 failed to find statistically significant associations between implants and connective tissue diseases.
Today, the FDA takes what feels like a middle-ground approach – allowing silicone implants on the market while requiring stringent monitoring and openly acknowledging both well-established local complications and rare but serious risks like BIA-ALCL. Their safety communication provides a comprehensive overview of known risks.
Current Research Insights & Contested Evidence
The scientific picture continues to evolve. Recent laboratory studies showing silicone-induced cell death provide potential mechanisms for harm, but population-based epidemiological studies still show relative risks close to 1.0 (indicating no increased risk) for most systemic diseases.
This disconnect between what happens in the lab, what patients report experiencing, and what large population studies show remains at the heart of ongoing scientific debate. Some researchers suggest that certain genetic or immune factors might make specific individuals more susceptible to silicone effects.
Legal Landscape: From Dow Corning to Allergan MDL
The story of breast implant lawsuit silicone toxicity litigation spans nearly four decades, with dramatic twists that have affected hundreds of thousands of women.
It all began in 1984 when Maria Stern won her groundbreaking case against Dow Corning. What started as a trickle soon became a flood, with thousands of individual lawsuits pouring into courts by the early 1990s. The federal court system created a multidistrict litigation (MDL) to manage the growing crisis.
Then came 1992 – a pivotal year when the FDA restricted silicone implants pending further safety studies. This regulatory action, combined with testimony before Congress and intense media coverage, created the perfect storm for litigation to explode.
By 1994, manufacturers proposed a massive $4.25 billion global settlement intended to compensate approximately 250,000 women. But the settlement buckled when claims exceeded expectations, ultimately driving Dow Corning to seek bankruptcy protection in 1995.
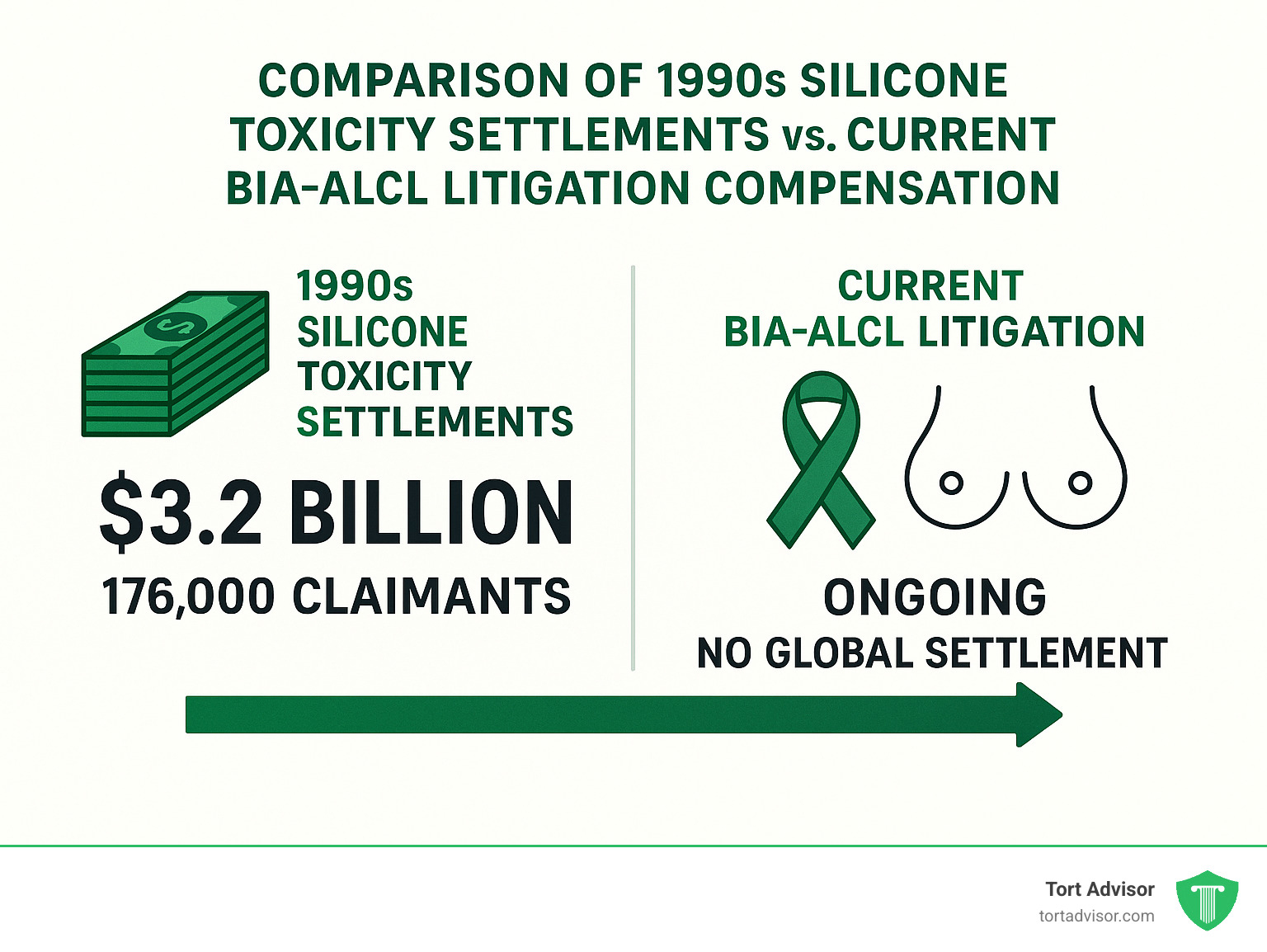
After years of negotiation, a revised settlement emerged with Dow Corning agreeing to pay $3.2 billion to compensate 176,000 women. Other manufacturers including Bristol-Myers Squibb, Baxter Healthcare, and 3M reached their own separate settlements with plaintiffs.
The legal winds shifted dramatically after 1999, when the Institute of Medicine published its influential report finding no convincing link between implants and systemic diseases. The impact was immediate – manufacturers began winning about 80% of cases that went to trial.
Today’s litigation landscape focuses primarily on breast implant-associated anaplastic large cell lymphoma (BIA-ALCL), particularly connected to Allergan’s Biocell textured implants. In 2019, the FDA requested a recall of these products after finding they posed a disproportionate risk.
Major Class Action Settlements & Compensation
The financial compensation available to women harmed by breast implants has varied dramatically depending on when and how they filed their claims.
The Dow Corning Settlement established a $3.2 billion fund for 176,000 women and created a 30-year program designed to address both current and future complications. Women with documented diseases could receive tiered payments based on severity, while those needing implant removal could access up to $5,000 in explant assistance.
In contrast, the current Allergan BIA-ALCL litigation remains ongoing with no global settlement yet reached. Allergan’s initial warranty programs offered just $1,000 for diagnostic tests and $7,500 for BIA-ALCL surgery – amounts widely criticized as inadequate given the life-threatening nature of this cancer.
Types of Claims: Strict Liability, Negligence, Failure to Warn
Women pursuing breast implant lawsuit silicone toxicity cases typically build their legal arguments on several key theories.
Strict liability claims don’t require proving the manufacturer was careless – only that the product was unreasonably unsafe and caused harm without substantial modification.
Negligence claims require showing the manufacturer breached its duty of care, and that this breach directly caused the plaintiff’s injury. This involves demonstrating the company failed to exercise reasonable care in designing, manufacturing, or testing its implants.
Failure to warn allegations focus on whether risks were adequately disclosed to patients. These claims often become particularly powerful when internal company documents reveal manufacturers knew about dangers but didn’t properly alert physicians or patients.
Breach of warranty claims assert that implants didn’t perform as guaranteed, whether through explicit promises or basic expectations of safety.
Role of Experts & “Silicone Doctors”
Expert testimony sits at the heart of breast implant lawsuit silicone toxicity litigation, with both sides heavily dependent on medical and scientific experts to establish or refute causation.
During the peak of 1990s litigation, a controversial phenomenon emerged – certain physicians became known as “silicone doctors” who frequently testified for plaintiffs. Some of these specialists treated thousands of women with alleged implant-related conditions and received substantial referrals from plaintiffs’ attorneys.
These practices raised ethical concerns. The American Medical Association specifically addressed physician testimony in its Opinion 9.07, emphasizing that doctors must testify honestly and avoid financial arrangements that might create conflicts of interest.
As courts became more sophisticated in evaluating scientific evidence, judges grew increasingly skeptical of expert testimony unsupported by peer-reviewed research. Many cases were dismissed after judges excluded plaintiffs’ experts under the Daubert standard, which requires scientific testimony to be both relevant and reliable.
Filing Your Claim: Deadlines, Eligibility & Strategy
Thinking about filing a claim for harm from your silicone breast implants? Your path forward will depend on several factors: when you received your implants, what specific injuries you’ve experienced, and which settlement funds or litigation options are currently available.
Timing matters tremendously in these cases. Each state has different statutes of limitation for product liability claims—typically ranging from 1-6 years from when you finded your injury. This “findy rule” is particularly important with breast implant lawsuit silicone toxicity cases, as complications often develop years after your initial surgery.
To build a strong claim, you’ll need solid documentation. This includes medical records confirming you received silicone implants, evidence of complications through imaging studies and surgical reports, a clear timeline of your symptoms and treatments, and expert medical opinions connecting your condition to your implants.
Your medical records tell your story. Make sure you gather comprehensive documentation including your original implantation surgical records, all subsequent imaging (MRIs, ultrasounds, mammograms), detailed records of symptoms and treatments, and if applicable, explantation surgical reports from when your implants were removed.
If you’re considering removing your implants, talk to both medical and legal professionals before proceeding. While explantation might be medically necessary and can provide important evidence for your case, the timing can significantly impact both your health outcomes and the strength of your legal claim.
Breast Implant Lawsuit Timelines & Requirements
Different litigation pathways come with specific deadlines and requirements:
For the SF-DCT (Dow Corning Settlement), most deadlines have now passed since this settlement was established in the 1990s. The final disease claim filing deadline was June 3, 2019, while explant assistance claims had to be filed by June 2, 2014. However, the settlement fund continues processing previously filed claims.
The Allergan BIA-ALCL MDL represents currently active litigation. It uses a streamlined short-form complaint process, though state statutes of limitation still apply. You’ll need documentation proving you received Allergan textured implants and have a BIA-ALCL diagnosis.
Individual lawsuits remain an option, especially for recent injuries or newly finded complications. These are subject to your state’s specific statutes of limitation and can proceed in state or federal court depending on your circumstances.
Checking Claim Status & Getting Help
Already filed a claim? Several resources can help you check its status:
For SF-DCT Claims, visit www.sfdct.com and enter your Settlement ID number, call their helpline at 1-866-874-6099, or contact the appropriate claims office for your specific claim type.
If you filed under MDL-926, visit the official MDL website, use your claim number to check status, or contact the claims administrator directly with questions.
For current litigation, your attorney should provide regular updates. Court websites often post MDL status reports, and case management orders outline upcoming deadlines and proceedings.
At Tort Advisor, we connect clients with experienced attorneys who specialize in breast implant lawsuit silicone toxicity cases. These specialists can provide personalized guidance custom to your unique situation. More info about breast-implant services
Maximizing Your Recovery
To strengthen your claim and potentially increase your compensation, consider these practical steps:
Document symptoms carefully by keeping a daily journal of physical symptoms, emotional impacts, and limitations on your activities. Obtain appropriate imaging—regular MRIs can detect silent ruptures and provide crucial evidence for your case.
Track all expenses carefully, including medical costs, travel expenses for treatments, lost wages, and other financial impacts. Join support groups to connect with other implant recipients who can share valuable information and resources.
Consider all potential claims—you may have grounds for action against manufacturers, surgeons, and healthcare facilities, depending on your specific circumstances.
Frequently Asked Questions about Silicone Implant Litigation
Do silicone implants cause autoimmune disease or cancer?
When it comes to silicone implants and health concerns, the scientific evidence paints a complex picture.
For autoimmune diseases, the major scientific panels have spoken quite clearly. Both the Institute of Medicine and the American College of Rheumatology have concluded there isn’t enough evidence to say silicone implants cause conditions like lupus or rheumatoid arthritis. The large population studies consistently show relative risks hovering around 1.0 – indicating no increased risk.
That said, cancer concerns tell a different story. While there’s no evidence linking silicone implants to breast cancer itself, we now have compelling data connecting textured implants (especially Allergan’s Biocell line) with a rare cancer called breast implant-associated anaplastic large cell lymphoma (BIA-ALCL). With over 700 cases worldwide and 36 deaths, the FDA has determined that Allergan’s textured implants carry about six times greater risk compared to other textured varieties.
Perhaps most interesting is the phenomenon many women call “Breast Implant Illness.” Though not officially recognized as a medical diagnosis, thousands of women report a cluster of symptoms including fatigue, joint pain, and cognitive fog. Recent lab studies showing silicone can trigger cell death pathways might explain why many of these women experience improvement after having their implants removed.
What compensation can I expect from a successful claim?
Compensation in breast implant lawsuit silicone toxicity cases varies tremendously based on several key factors.
First, the nature and severity of your injury matters enormously. Cases involving BIA-ALCL typically command much higher settlements than claims based on local complications or contested systemic symptoms. The strength of causation evidence also plays a crucial role – claims with clear scientific support linking the implant to your specific injury tend to result in better outcomes.
Looking at historical settlements provides some perspective. The massive 1990s Dow Corning settlement worked out to about $18,800 per claimant on average. Individual verdicts have ranged much higher – from $5.4 million in the 1991 Toole case to $25 million in the Johnson case that same year.
For current BIA-ALCL litigation, experts anticipate settlements might range from $150,000 to $500,000 per plaintiff, though these numbers remain speculative until more cases resolve.
These figures don’t account for attorneys’ fees (typically 33-40% of your recovery) or medical liens that may need to be satisfied from your settlement.
Should I remove my implants before filing suit?
This question touches on both medical and legal considerations, and deserves thoughtful reflection.
From a medical standpoint, you should only remove implants if it’s necessary for your health or recommended by your doctor. Explantation carries its own risks – scarring, infection, and cosmetic changes among them. For diagnosed BIA-ALCL, prompt removal of implants and surrounding tissue is the standard treatment and shouldn’t be delayed.
Legally speaking, explantation can provide valuable evidence, especially if your surgeon finds rupture or leakage. The condition of removed implants may strengthen causation arguments in your case, and medical documentation showing the necessity for removal can bolster your damages claims.
The FDA offers balanced guidance here: don’t remove implants if you have no symptoms, consider regular monitoring with MRI or ultrasound to detect silent ruptures, and have a thorough risk-benefit conversation with your healthcare provider.
My strongest recommendation? Consult both medical and legal professionals before making this decision. Your health should always be the primary consideration, with legal strategy as a secondary factor.
More info about breast-implant lawsuits
Conclusion
Navigating the complex landscape of breast implant lawsuit silicone toxicity requires understanding both the evolving science and the legal framework that has developed over four decades of litigation.
The scientific evidence presents a mixed picture. While large epidemiological studies haven’t confirmed links between silicone implants and most systemic diseases, laboratory research continues to uncover potential mechanisms of harm. Local complications like capsular contracture and rupture are well-documented, and specific risks like BIA-ALCL have strong evidence bases, particularly for textured implants.
The legal landscape reflects this scientific uncertainty. The massive class actions of the 1990s resulted in billions in settlements despite contested causation evidence. Today’s litigation focuses more narrowly on specific complications with stronger scientific support, particularly BIA-ALCL linked to textured implants.
If you’re considering your options after experiencing complications, documentation is crucial. Keep every medical record related to your implantation, symptoms, treatments, and any explantation procedure. These records will form the backbone of any potential claim.
Timing matters too. Each state has different statutes of limitation, and settlement funds have specific filing deadlines that can’t be missed.
The path forward isn’t one you should walk alone. Expert guidance is essential in these cases where medicine and law intersect in complicated ways. Both specialized healthcare providers and experienced attorneys can help you steer these complex waters.
Individual circumstances vary significantly. Your specific implant type, the symptoms you’ve experienced, your medical history, and even where you live all affect your potential legal remedies.
These lawsuits highlight the challenges of establishing causation for complex medical conditions. They also show how litigation can drive both regulatory change and scientific inquiry, ultimately leading to safer medical devices for everyone.
If you believe you’ve been harmed by silicone breast implants, we at Tort Advisor can connect you with specialized attorneys experienced in these complex cases. With representation in all 50 states, we ensure you receive guidance custom to your specific situation and jurisdiction.
For more information about breast implant lawsuits and your legal options, visit our comprehensive resource center.
The journey through breast implant lawsuit silicone toxicity litigation isn’t easy, but with the right support and information, you can make informed decisions about your health and legal rights.
Free Confidential Case Evaluation
Complete the short form below to get an immediate FREE case review with an expert in your specific claim. Don't wait, your case could be time sensitive to file a claim.
Related Posts
Learn how to qualify for an Uber sexual assault lawsuit: MDL 3084 criteria, evidence, deadlines & compensation steps.
Consult cosmetic injury lawyers for botched procedures. Learn negligence proof, damages, & claims against surgeons today!
Understand your rights after a California wildfire. Learn how to file a wildfire lawsuit California, claim compensation, and hold utilities accountable.
Find a top Mississippi accident lawyer. Get immediate steps, state laws, compensation tactics & max recovery after your crash.
Discover New Jersey disability benefits: TDI, FLI, SSDI, SSI rates, eligibility, applications & appeals for 2025-2026.
Hire a Depo-Provera lawsuit attorney now. Fight Pfizer for meningioma risks from injections. Free consult, MDL updates & settlements up to $1.5M.