

Understanding the Growing Wave of Depo-Provera Legal Action
A depo-provera lawsuit is a legal claim filed by women who developed serious health issues, most notably brain tumors called meningiomas, after using the birth control injection. Here’s a summary of the situation:
Key Points About Depo-Provera Lawsuits:
- The Main Allegation: Manufacturer Pfizer failed to adequately warn users about the increased risk of meningioma brain tumors.
- Current Status: Over 1,200 federal lawsuits have been consolidated in Florida (MDL No. 3140) as of October 2025.
- Scientific Evidence: A 2024 study found women using Depo-Provera for over a year had a 5.6 times higher risk of developing meningiomas.
- Who May Qualify: Women who used Depo-Provera for at least one year and were later diagnosed with a meningioma.
- Legal Basis: Claims include failure to warn, design defect, and negligence against Pfizer.
- International Context: European and Canadian labels warn of meningioma risk, but U.S. labels do not.
The human cost is significant. One plaintiff, Robin Phillip, used Depo-Provera for nearly 30 years before being diagnosed with a brain tumor at age 37. Her treatment required two major brain surgeries, left her with vision loss, and forced her to relearn how to walk. “I would never have taken the shot if I had known about the risk,” she stated.
Depo-Provera, an FDA-approved contraceptive since 1992, is administered via injection every three months. While millions of women have used it for its convenience, recent scientific studies have uncovered a disturbing link between the drug and serious neurological harm. This guide will help you understand the legal landscape, the science, and your options if you’ve been affected.
What is Depo-Provera and Why is it Facing Legal Scrutiny?
Depo-Provera is a popular birth control shot approved by the FDA in 1992. Its active ingredient, medroxyprogesterone acetate (MPA), is a synthetic hormone that prevents pregnancy by stopping ovulation, thickening cervical mucus, and thinning the uterine lining. The shot is administered every three months, a convenience that has made it a choice for millions of women. It is also prescribed for conditions like endometriosis.
The legal scrutiny arises from what its manufacturers—Pfizer and its subsidiary Pharmacia & Upjohn—allegedly failed to disclose. Lawsuits claim these companies did not adequately warn users about the risk of serious health dangers, particularly brain tumors. This alleged failure to warn is the foundation of every depo-provera lawsuit. For more on the health concerns, our Depo-Provera Severe Side Effects guide offers details.
The Primary Allegation: Meningioma Brain Tumors
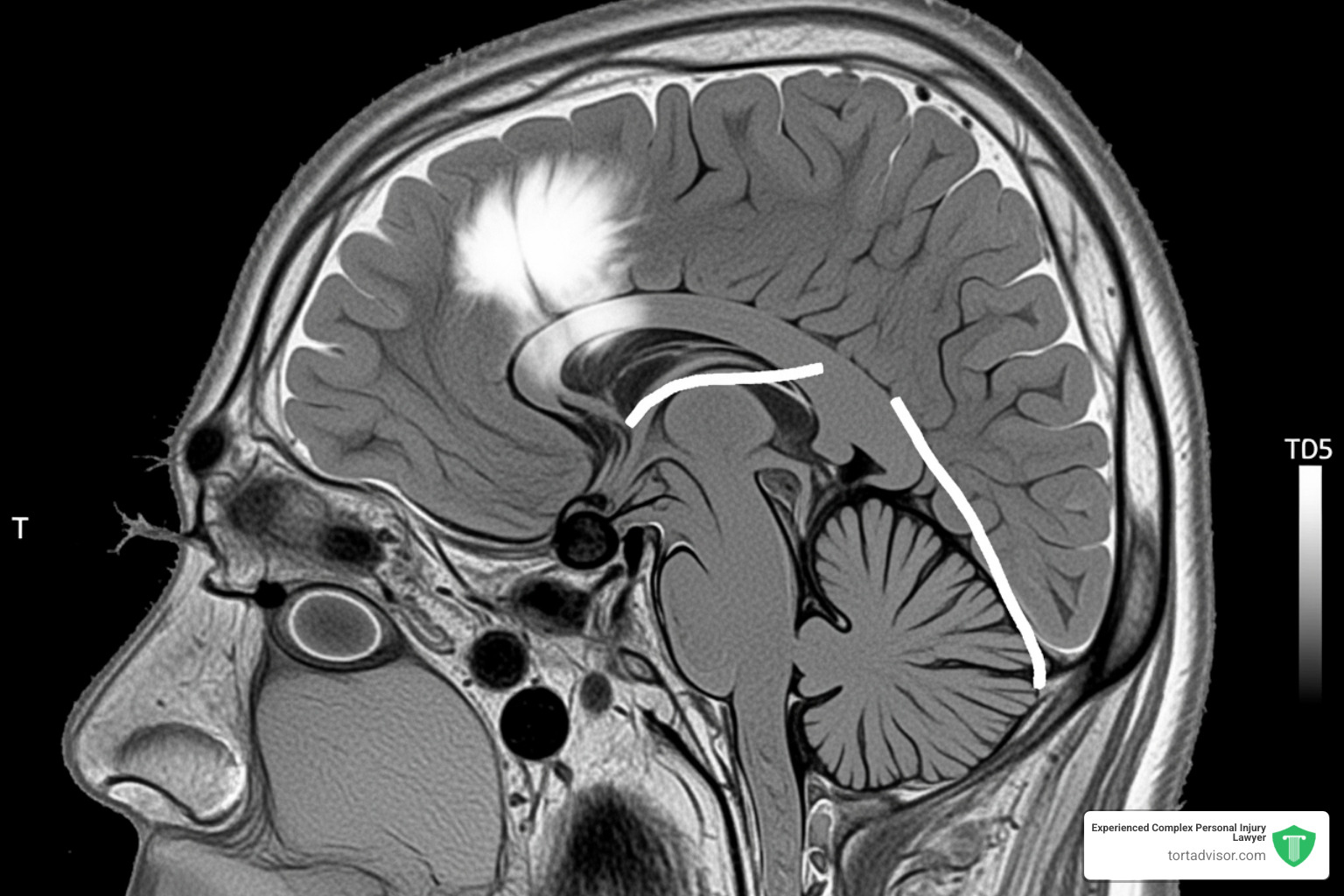
The most serious claim involves meningiomas, tumors that grow in the protective tissues surrounding the brain and spinal cord. These are the most common type of primary brain tumor and occur more often in women, suggesting a hormonal link.
While most meningiomas are non-cancerous, their growth inside the skull can cause severe problems by pressing on the brain. Symptoms can be life-altering and include:
- Severe, persistent headaches
- Vision problems (blurred or double vision)
- Seizures
- Hearing or balance issues
- Cognitive difficulties
Treatment often involves a craniotomy, a major brain surgery to remove the tumor. Some patients, like Robin Phillip, have required multiple surgeries and been left with permanent disabilities like vision loss. These are not minor side effects; they are devastating injuries. The link between Depo-Provera and these tumors is the central issue driving the litigation. Our Depo Shot Brain Tumor page explores this connection further.
Other Alleged Side Effects
While meningiomas are the focus of current lawsuits, other serious health concerns have been linked to Depo-Provera.
- Bone Density Loss: The risk of significant bone loss is so well-documented that in 2004, the FDA required a black box warning—its strongest caution—advising against use for more than two years. This issue led to a Canadian class action that Pfizer settled. Learn more at our Depo-Provera Bone Loss Lawsuit page.
- Pseudotumor Cerebri: Also known as intracranial hypertension, this condition involves increased pressure around the brain, mimicking a tumor’s symptoms and potentially causing permanent vision loss.
- Blood Clots: Some cases have also raised concerns about an increased risk of blood clots.
The common thread in these allegations is that women were not properly warned about serious risks, denying them the ability to make an informed choice about their health.
The Scientific Link: What Research Reveals About Depo-Provera and Meningioma
The depo-provera lawsuit litigation is supported by compelling scientific evidence. The biological mechanism is plausible: meningioma tumors often have progesterone receptors on their surface. The synthetic hormone in Depo-Provera, medroxyprogesterone acetate (MPA), may bind to these receptors and stimulate tumor growth.
While this hormonal connection has been studied for decades, several recent large-scale studies have solidified the link:
- The 2024 BMJ Study: This landmark French study, published in The British Medical Journal, found that women who used Depo-Provera for more than one year had a 5.6 times higher risk of developing a meningioma. You can review the findings at The BMJ study.
- The JAMA Neurology Study: Researchers analyzing data from over ten million women found a twofold increase in meningioma risk among Depo-Provera users. The study, published in JAMA Neurology, noted the risk was higher for women who used the shot for over four years. Explore the research at JAMA Neurology study.
- University of British Columbia Study: Another study found a 3.55-fold increased risk of meningioma for women who used Depo-Provera for more than a year.
The consistency across these independent, large-scale studies is striking. While the overall risk of developing a meningioma is low, this increased risk is significant. For example, one analysis suggests that for every 1,111 women using Depo-Provera for three years, one additional brain tumor could be expected.
Although these are observational studies that show strong associations rather than proving direct causation, their combined weight provides a powerful scientific foundation for the legal claims. This evidence transforms individual stories of harm into a clear, research-backed pattern. For more on how these findings support legal claims, see our Depo-Provera Lawsuit Side Effects Guide.
The Current State of the Depo-Provera Lawsuit Litigation
The depo-provera lawsuit litigation is large and growing rapidly. As of October 2025, more than 1,200 federal lawsuits have been filed against Pfizer and its related companies. The core allegation is that these companies failed to warn patients and doctors about the risk of meningioma brain tumors associated with Depo-Provera.
To manage these cases efficiently, they have been consolidated into a multidistrict litigation (MDL). For the latest information on the litigation’s progress, you can visit our Depo-Provera Lawsuit Updates 2025 page.
The Depo-Provera MDL (Multidistrict Litigation)
The official proceeding, MDL No. 3140, is centralized in the U.S. District Court for the Northern District of Florida under Judge M. Casey Rodgers, who is experienced in complex pharmaceutical cases. You can find official court filings here: Depo-Provera (Depot Medroxyprogesterone Acetate) Products Liability Litigation | Northern District of Florida.
An MDL is not a class action. Here are the key differences:
- Individual Lawsuits: In an MDL, each plaintiff retains their own individual lawsuit. In a class action, one lawsuit represents the entire group.
- Individual Compensation: MDL settlements or verdicts are based on the specific damages of each person (medical bills, lost wages, etc.). Class action payouts are typically divided among all members from a single lump sum.
- More Control: Plaintiffs in an MDL have more control over their case and can reject settlement offers.
This structure is ideal for Depo-Provera cases because the injuries vary so widely. The MDL will streamline pretrial activities like evidence gathering. A few representative cases, known as bellwether trials, will likely be tried first to help establish settlement values for the remaining lawsuits. In addition to the federal MDL, dozens of cases are also pending in New York state courts. For more on the distinction, see our Depo-Provera Class Action Lawsuit 2024 page.
Filing a Claim: What Potential Plaintiffs Need to Know
If you used Depo-Provera and were diagnosed with a meningioma, you may be wondering if you can file a depo-provera lawsuit. While every case is different, there are general criteria and steps to consider.
Do I Qualify?
Generally, you may have a claim if you meet these three criteria:
- Significant Use: You used Depo-Provera for at least one year (approximately four injections).
- Confirmed Diagnosis: You were diagnosed with a meningioma brain or spinal tumor.
- Timeline: Your diagnosis occurred after you began using the injections, establishing a plausible connection.
For more details, see our page on Who May Qualify to File a Depo Provera Lawsuit?.
What Evidence is Needed?
Building a strong case requires documentation. You will need to gather:
- Proof of Use: Medical or pharmacy records showing you received Depo-Provera injections.
- Proof of Injury: All medical records related to your meningioma diagnosis and treatment, including MRIs, CT scans, pathology reports, and surgical notes.
Understanding the Legal Basis for a Depo-Provera Lawsuit
These lawsuits are based on product liability law, with failure to warn being the central claim. Plaintiffs argue that Pfizer knew or should have known about the meningioma risk but failed to inform U.S. patients and doctors. This claim is strengthened by the fact that warnings were added to Depo-Provera labels in other countries, including Canada and in Europe, following a recommendation from the European Medicines Agency. The lack of a similar warning in the U.S. suggests a failure to protect American patients.
Other legal claims include design defect (arguing the drug is inherently unsafe) and negligence (claiming the manufacturer was careless).
Statute of Limitations and the Discovery Rule
There is a limited time to file a lawsuit, known as the statute of limitations. This deadline varies by state, often ranging from one to three years. However, the clock doesn’t always start when the injury occurs. Under the “discovery rule,” the deadline may begin when you discovered, or reasonably should have discovered, the link between your meningioma and Depo-Provera. For many women, this discovery is recent. Because these deadlines are strict and complex, it is crucial to speak with an attorney immediately to protect your rights. Our Depo-Provera Lawsuit Criteria Guide has more on filing requirements.
Potential Compensation in a Depo-Provera Lawsuit
A successful depo-provera lawsuit can provide compensation for the significant financial, physical, and emotional damages caused by a meningioma diagnosis. While no global settlement has been reached, plaintiffs can seek recovery for several types of damages.
- Economic Damages: This includes all past and future medical expenses, from brain surgery and hospital stays to rehabilitation and medication. It also covers lost wages and any reduction in your future earning capacity.
- Non-Economic Damages: This compensates for intangible losses, such as physical pain and suffering, emotional distress, anxiety, and loss of quality of life (the inability to enjoy hobbies and daily activities).
- Punitive Damages: In some cases, courts may award punitive damages. These are intended to punish the manufacturer for reckless conduct, such as hiding known risks, and to deter similar behavior in the future.
For a more detailed breakdown, see our Depo-Provera Lawsuit Compensation Guide 2025.
Factors Influencing Settlement Amounts
Compensation is not one-size-fits-all; it is custom to the specifics of your case. Key factors that influence settlement values include:
- Severity of the Injury: A large, aggressive tumor requiring multiple surgeries will lead to a higher valuation than a small, easily removed one.
- Long-Term Prognosis: Permanent disabilities like vision loss, seizures, or cognitive impairment significantly increase a case’s value.
- Duration of Depo-Provera Use: Longer use strengthens the link to the drug, as scientific evidence shows a dose-dependent risk.
- Strength of Evidence: Clear and complete medical records are crucial.
- Age of the Plaintiff: A younger person with a longer period of lost earning capacity may receive a larger award.
While it’s too early for exact figures in the Depo-Provera MDL, legal experts project that settlements could range from $100,000 to over $500,000, with the most severe injury cases potentially exceeding that. You can get a preliminary estimate with our Calculator: Depo Provera Settlement Calculator, but only an experienced attorney can provide an accurate assessment.
Frequently Asked Questions about Depo-Provera Lawsuits
When considering a depo-provera lawsuit, many questions arise. Here are answers to some of the most common concerns.
What is the FDA’s role in the Depo-Provera litigation?
The FDA’s role is a key issue. Pfizer applied to the FDA to add a meningioma warning to the U.S. label, but the FDA denied the request, citing insufficient evidence from observational studies. Pfizer is now using this denial as a “federal preemption” defense, arguing it cannot be sued under state law for failing to add a warning that the FDA rejected.
However, plaintiffs’ lawyers argue that Pfizer had other ways to warn patients, such as using the “Changes Being Effected” (CBE) process to update the label independently. The most compelling counterargument is the international double standard: regulators in Europe, Canada, and South Africa have all moved to add meningioma warnings. The European Medicines Agency and South Africa’s drug regulatory agency have both issued relevant updates. This suggests Pfizer could have, and should have, done more to warn American women. Learn more about similar cases on our Birth Control Shot Lawsuit page.
What steps should I take if I believe I’ve been harmed by Depo-Provera?
If you suspect your meningioma is linked to Depo-Provera, take these steps immediately:
- Seek Medical Care: Your health is the priority. Discuss your symptoms and Depo-Provera history with your doctor.
- Gather Records: Collect all medical and pharmacy records related to your Depo-Provera use and your tumor diagnosis and treatment.
- Document Your Experience: Keep a journal detailing your symptoms, pain levels, and how the condition has impacted your daily life.
- Consult an Attorney: Speak with a lawyer specializing in pharmaceutical lawsuits as soon as possible to protect your legal rights before the statute of limitations expires.
- Avoid Social Media: Do not post about your health or legal case online, as it could be used against you.
What are the benefits of hiring a lawyer for a Depo-Provera lawsuit?
Facing a pharmaceutical giant like Pfizer alone is nearly impossible. An experienced lawyer provides critical advantages:
- Expertise: They understand the complex science and legal procedures of mass tort litigation.
- Resources: They have access to medical experts needed to prove your case.
- Protection: They handle all communication with the defendants, shielding you from pressure.
- Maximizing Compensation: They have the negotiation skills to secure the full value of your claim.
- No Financial Risk: These lawyers work on a contingency fee basis, meaning you pay nothing unless they win your case.
An experienced attorney levels the playing field and allows you to focus on your recovery. Our Depo-Provera Injury Lawyer page has more on finding the right legal help.
How to Get Help for Your Depo-Provera Claim
Taking on a pharmaceutical giant like Pfizer requires specialized legal representation. If you were diagnosed with a meningioma after using Depo-Provera, you deserve an advocate with the resources and experience to fight for the compensation you are owed.
These cases are complex, involving detailed medical evidence and powerful corporate legal teams. You need an attorney who can level the playing field.
That’s where Tort Advisor can help. We connect individuals with top-rated attorneys who have proven track records in handling depo-provera lawsuit cases. The lawyers in our network are highly skilled in product liability litigation and have successfully represented clients in similar pharmaceutical injury claims.
When you reach out to us, we will listen to your story and help you understand your legal options. We can connect you with an experienced attorney for a free, no-obligation case evaluation.
You are not just a case number. You are someone who trusted a medication and suffered life-altering consequences. Time to file a claim is limited, so don’t wait to seek justice and accountability. Contact us today to take the first step.
Free Confidential Case Evaluation
Complete the short form below to get an immediate FREE case review with an expert in your specific claim. Don't wait, your case could be time sensitive to file a claim.
Related Posts
Did a North Dakota product cause harm? Understand product liability, your rights, and how to take action for defects.
Get justice for clergy abuse. Find an expert Priest abuse lawyer to navigate complex laws and hold institutions accountable.
Diagnosed with meningioma after Depo-Provera? Understand potential Depo-Provera lawsuit settlements, risks, & how to claim compensation.
Uncover the truth about uber sexual assault cases. Learn about the alarming scale, Uber's accountability, and legal options for justice.
Facing wildfire losses? Discover the best wildfire lawsuit attorneys in California to fight for your full recovery and justice.
Exposed to Roundup & diagnosed with NHL? Discover how to sue Monsanto, understand eligibility, & seek compensation. Your guide to justice.