

Understanding Your Rights After a Depo-Provera Diagnosis
A depo provera injury claim may be available if you developed a meningioma brain tumor after using this popular birth control shot. Here’s what you need to know:
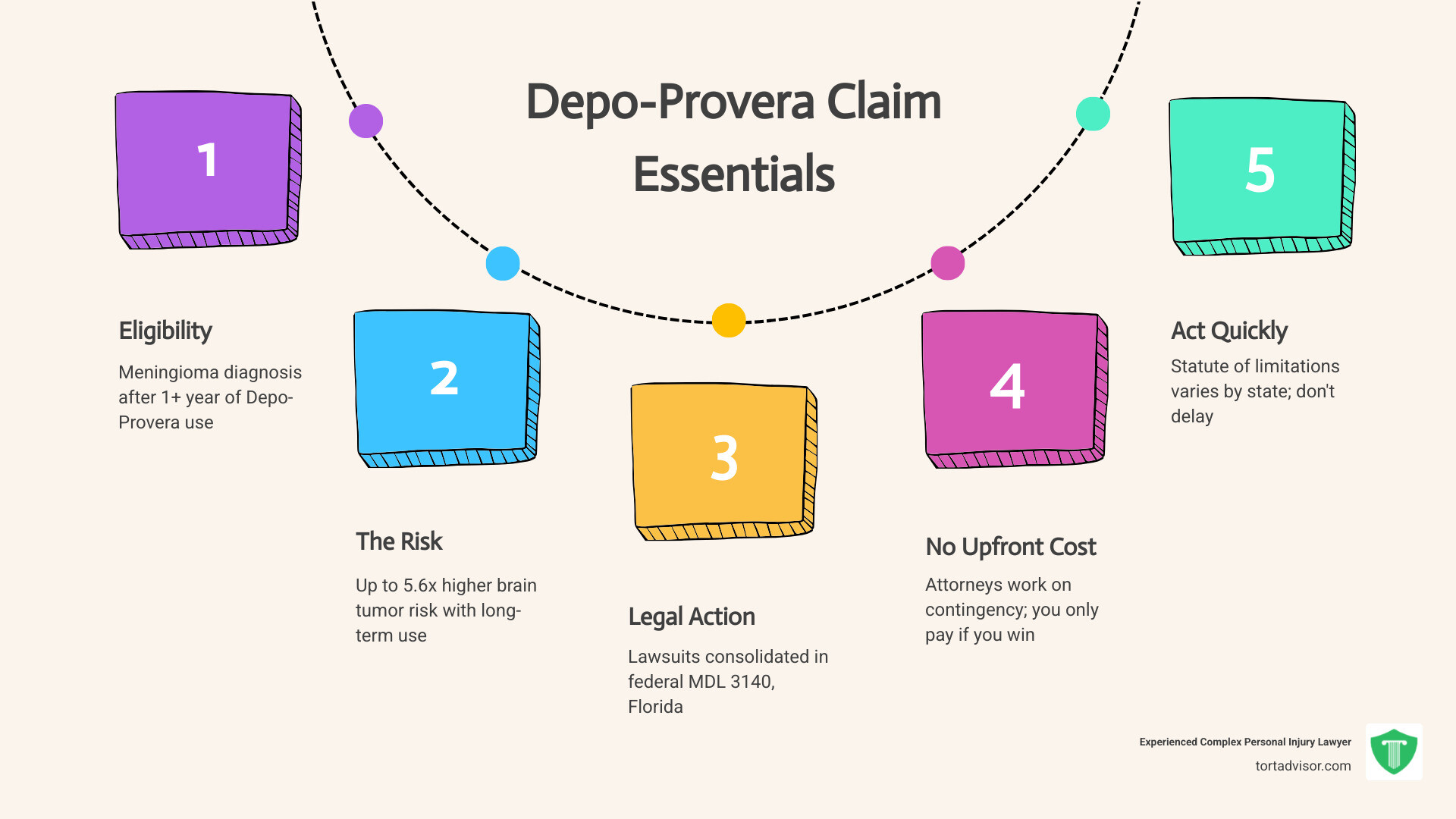
Quick Facts About Depo-Provera Claims:
- Who Qualifies: Women diagnosed with meningioma after using Depo-Provera for one year or more
- The Risk: Studies show up to 5.6 times higher risk of brain tumors with long-term use
- Legal Action: Lawsuits consolidated in federal MDL 3140 in Florida
- No Upfront Costs: Attorneys work on contingency—you only pay if you win
- Time Limits: Statute of limitations varies by state, so act quickly
Depo-Provera has been prescribed to millions of women as a convenient contraceptive injection administered every 12 weeks. But recent scientific studies have revealed a troubling connection between this birth control shot and an increased risk of developing meningioma brain tumors—particularly among women who used it for extended periods.
The central issue: Pfizer, the manufacturer, allegedly knew about these risks but failed to adequately warn patients and doctors in the United States, even while updating warning labels in other countries like Canada.
If you or someone you love developed a brain tumor after using Depo-Provera, you’re not alone. Thousands of women are now stepping forward to hold Pfizer accountable through product liability lawsuits. These claims seek compensation for medical bills, lost wages, pain and suffering, and the devastating impact these tumors have had on daily life.
My name is Mason Arnao, and while my background is in technology and SaaS architecture, I’ve dedicated myself to helping individuals steer complex legal processes by connecting them with experienced attorneys who specialize in areas like depo provera injury claims. Understanding your legal rights is the first step toward getting the justice and compensation you deserve.
The Science Behind Depo-Provera Health Risks
Understanding the science behind depo provera injury claims means knowing what the medication does and what research has revealed about its risks.
What is Depo-Provera and How Does It Work?
Depo-Provera is the brand name for medroxyprogesterone acetate (MPA), a synthetic version of the hormone progestin. It’s a popular contraceptive injection often called “the shot.”
It works by preventing ovulation (the release of an egg), thickening cervical mucus to block sperm, and thinning the uterine lining to prevent a fertilized egg from implanting.
The injection is administered every 12 weeks—roughly every three months—usually in your arm or buttocks. Approved by the FDA in 1992, its convenience made it popular; nearly one in four sexually active U.S. women used it between 2015 and 2019.
Beyond birth control, doctors also prescribe Depo-Provera to treat menstrual disorders, endometriosis, and various hormonal imbalances. Its widespread use made it a trusted option until recent studies raised serious safety concerns.
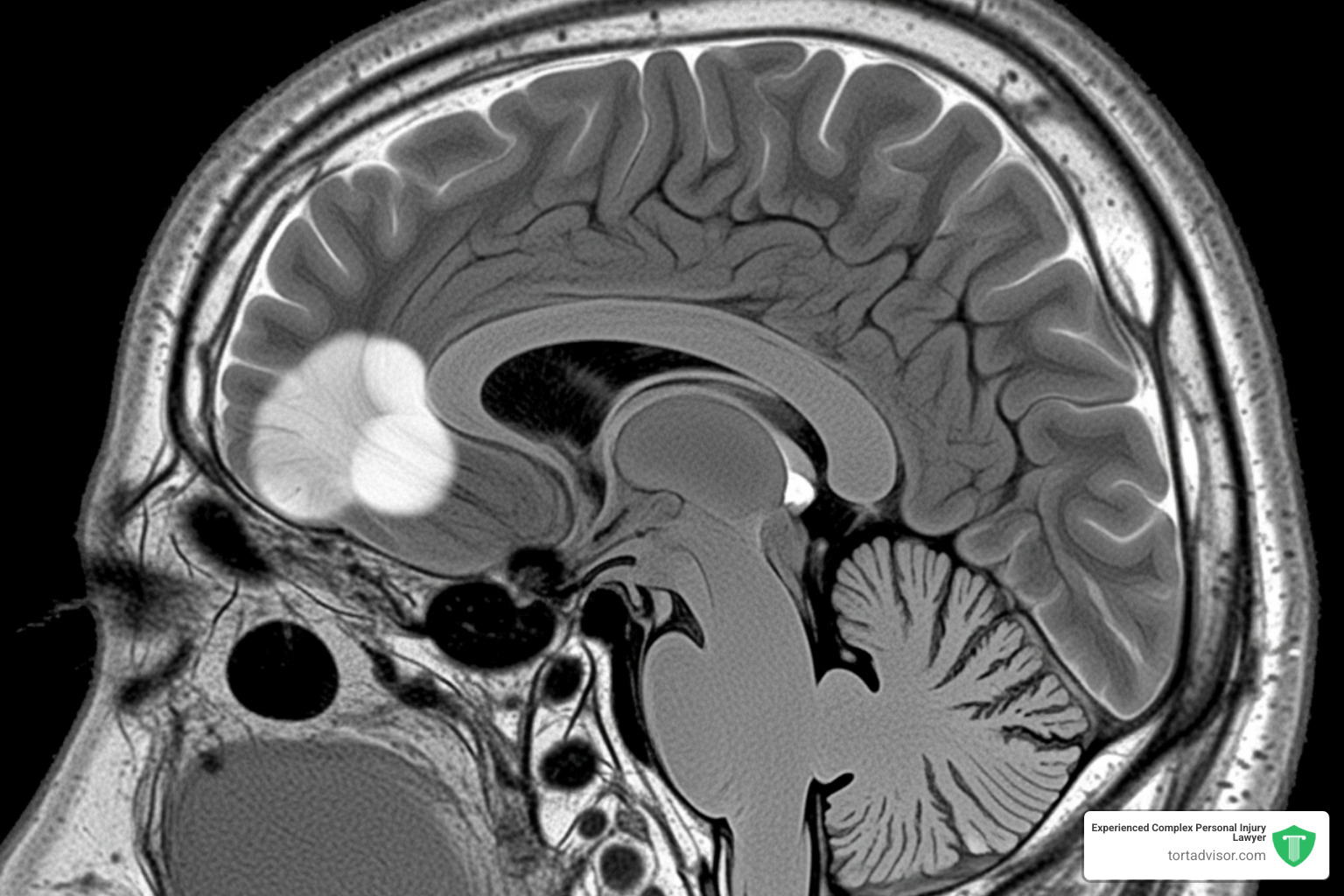
What is a Meningioma Brain Tumor?
A meningioma is a tumor that grows in the meninges—the protective membranes wrapping your brain and spinal cord. When a tumor forms there, it’s called a meningioma.
These tumors are the most common type of primary brain tumor, accounting for about 40% of all diagnoses. Most meningiomas are benign (non-cancerous) and grow slowly. However, even a benign tumor can cause serious problems if its size creates pressure against the brain, spinal cord, or nerves.
That pressure can trigger a range of neurological symptoms. What makes this particularly relevant to Depo-Provera users is that meningiomas occur more frequently in women than men, pointing to hormonal influences.
The Scientific Evidence Linking Depo-Provera to Meningiomas
The link between Depo-Provera and meningiomas is grounded in years of scientific research.
Since the 1980s, scientists have known that many meningioma cells have progesterone receptors. A 1991 study in the Journal of Neurosurgery showed that blocking progesterone could reduce tumor growth, raising questions about the effect of flooding the body with synthetic progesterone like that in Depo-Provera.
The answer came in recent landmark studies.
A 2024 study in the British Medical Journal analyzed data from over 18,000 women who underwent surgery for meningiomas. The findings were striking: women who used medications containing medroxyprogesterone acetate (like Depo-Provera) for more than one year faced a 5.6 times higher risk of developing an intracranial meningioma compared to non-users.
Then in 2025, researchers published another significant study in the JAMA Neurology journal titled “Depot Medroxyprogesterone Acetate and Risk of Meningioma in the US.” This Cleveland Clinic study of more than 10 million women found that long-term users of injectable MPA had almost 2.5 times the risk of developing a meningioma. The risk climbed even higher for women who started using it after age 31 or continued for more than four years.
Additional research has reinforced these findings. One 2024 study found that MPA increased the risk of intracranial meningioma requiring surgery by 5.6 times. Another showed that women who used Depo-Provera for over a year had a 3.5 times greater risk compared to women taking combination birth control pills.
Dr. Anjali R. Bansal, a neuro-oncologist, put it plainly in the Journal of Clinical Oncology: “Patients must be aware of the risks associated with long-term contraceptive use, especially those that may not have been adequately communicated in the past.”
These studies provide compelling evidence that Depo-Provera can cause brain tumors, particularly meningiomas. The consistency across multiple reputable publications makes the connection impossible to ignore.
Signs and Symptoms of a Meningioma
Because meningiomas grow slowly, symptoms can be subtle at first and easy to dismiss. As the tumor presses on the brain or spinal cord, symptoms become more severe and can seriously affect daily life.
If you’ve used Depo-Provera, watch for these warning signs: persistent headaches that are new or worsening, vision changes like blurriness or double vision, hearing loss or tinnitus (ringing in the ears), seizures without a prior history, and memory problems or confusion.
You might also notice weakness or numbness in your arms or legs, difficulty speaking, persistent nausea and vomiting, or dizziness and balance problems. Some women experience irregular periods or unexplained weight gain, which, when combined with neurological symptoms, warrant medical attention.
If you’re experiencing any of these symptoms, especially if they’re new or worsening, seek medical attention immediately. Meningiomas are typically diagnosed through an MRI. While many are treatable with surgery, some are inoperable and require radiation or other treatments, which can reduce quality of life. Early diagnosis offers the best chance for effective treatment and provides the strongest foundation for a depo provera injury claim.
Do You Qualify for a Depo-Provera Injury Claim?
Eligibility for a depo provera injury claim depends on several key factors. If you’ve been diagnosed with a meningioma after using Depo-Provera, you may have grounds for a lawsuit.
Who is Eligible to File a Lawsuit?
To have a strong case, your situation should generally match the following criteria:
First, you need a history of Depo-Provera use. This means you received at least two injections of the brand-name Depo-Provera, Depo-SubQ Provera, or an authorized generic version.
The most critical factor is a diagnosis of a meningioma or other brain tumor. Your diagnosis must have occurred during or after your use of the contraceptive to establish a potential link.
Research shows that long-term use creates the most significant risk. Women who used Depo-Provera for at least one year face substantially higher odds of developing meningiomas. If you used it for several years, your case is even stronger, as studies show the highest risks for women who used it for more than four years.
Cases involving surgical removal of the tumor or those causing significant neurological symptoms tend to carry more weight. If you’ve undergone brain surgery or are experiencing severe, life-altering symptoms like vision loss, seizures, or memory problems, these factors strengthen your claim.
Even if you used Depo-Provera years ago, you may still be able to file a claim. Many states apply the “findy rule” to the statute of limitations, meaning the deadline doesn’t start until you knew, or should have known, about your injury and its link to Depo-Provera. If you were recently diagnosed and just learned about the connection, your window to take legal action may still be open.
For a deeper dive into qualification criteria, I recommend checking out our detailed guides on Who May Qualify to File a Depo-Provera Lawsuit? and our comprehensive Depo-Provera Lawsuit Criteria Guide.
Proving Depo-Provera Caused Your Injury
Proving causation in a product liability lawsuit is complex, but you don’t have to do it alone. Experienced attorneys and their medical experts handle this process.
The legal standard is to show that Depo-Provera can cause meningiomas (which scientific studies establish) and that it’s more likely than not that it caused your specific tumor.
The foundation of a strong case is medical and pharmacy records. These documents create a timeline showing your Depo-Provera use, when symptoms began, and when you were diagnosed.
Next comes expert testimony from medical professionals like oncologists, neurologists, and epidemiologists. These experts explain how medroxyprogesterone acetate (MPA) can promote tumor growth by interacting with progesterone receptors on meningioma cells. They will reference the scientific study on causation and other research to build the case.
Finally, there’s the differential diagnosis process. Pfizer’s defense will argue that something else caused your tumor. Your legal team’s job is to systematically rule out these alternative explanations, using your medical history and expert opinions to show that Depo-Provera is the most likely cause.
The attorneys we connect you with understand this process and know how to build an airtight case that proves Pfizer’s product harmed you.
What About Generic Versions of Depo-Provera?
A common question is whether you can file a claim if you used a generic version of Depo-Provera. The answer is yes.
The lawsuits encompass generic versions containing medroxyprogesterone acetate (MPA) because the active ingredient and the risks are the same.
A legal principle called innovator liability, applicable in many states, allows claims against the original brand-name manufacturer (Pfizer) even if you used a generic. This is because generic manufacturers rely on the brand-name company’s research and warning labels. If Pfizer failed to adequately warn about the risks, it can be held responsible for injuries caused by generic versions.
Some generic versions are “authorized generics,” which are identical copies of Depo-Provera, sometimes made in Pfizer-owned facilities. Companies like Greenstone LLC and Prasco Labs are often named as defendants alongside Pfizer.
Whether you used brand-name Depo-Provera, Depo-SubQ Provera, or a generic, you may still have grounds for a claim. The key is the active ingredient and the failure to warn about its risks.
For more information about how these lawsuits are structured, visit our page on Depo-Provera Lawsuits.
Navigating the Legal Process Against Pfizer
Taking on a pharmaceutical giant like Pfizer can feel overwhelming, but you don’t have to do it alone. An experienced attorney can guide you through the legal process.
Your Legal Options: Filing a Lawsuit Against Pfizer
If you developed a meningioma after using Depo-Provera, you have the right to file a product liability lawsuit against Pfizer, the company that owns the rights to the contraceptive. These lawsuits center on a serious allegation: failure to warn.
The core of these claims is that Pfizer knew, or should have known, about the increased risk of brain tumors yet failed to adequately warn American women and their doctors. The evidence is striking: while Canadian and European labels for Depo-Provera have included warnings about meningiomas for years, the U.S. prescribing information remained silent on this risk. This suggests Pfizer knew the danger but failed to provide the same warnings to women in the U.S.
Lawsuits also allege negligence—that Pfizer was careless in the design, testing, or marketing of Depo-Provera—and strict product liability, which holds manufacturers responsible for harm caused by defective products.
Filing a depo provera injury claim against a major pharmaceutical corporation requires specialized legal expertise. Connecting with an experienced Depo-Provera Injury Lawyer is a critical step.
Current Status: The Depo-Provera MDL
Depo-Provera cases have been consolidated into a Multidistrict Litigation (MDL), which is different from a class action.
An MDL is not a class action. In a class action, everyone is grouped into one case. An MDL consolidates many individual lawsuits into one court for pretrial proceedings, but each case remains separate and retains its individual value. This is important because injuries vary in severity.
On February 7, 2025, the Judicial Panel on Multidistrict Litigation officially formed MDL 3140 IN RE: Depo-Provera (Depot Medroxyprogesterone Acetate) Products Liability Litigation. All federal Depo-Provera cases are now centralized in the U.S. District Court for the Northern District of Florida, overseen by Judge M. Casey Rodgers.
The MDL’s growth has been remarkable. As of October 22, 2025, there are 1,785 women who have filed lawsuits against Pfizer, with 1,346 cases in the federal MDL—a significant jump from just 435 in July. Plaintiffs’ lawyers report holding nearly 10,000 unfiled claims, meaning thousands more women may join the litigation.
As the MDL progresses, the court will likely select a few cases for bellwether trials. These test cases help both sides understand how juries might react to the evidence and often shape settlement negotiations for the remaining cases.
You can stay informed about the latest developments by visiting our Depo-Provera Lawsuit Updates 2025 page.
Statute of Limitations for a Depo-Provera Injury Claim
It’s critical to know that every legal claim has a deadline, called the statute of limitations. If you miss this deadline, you could lose your right to seek compensation, regardless of your case’s strength.
The statute of limitations varies by state, typically ranging from one to three years for product liability cases. Knowing your state’s specific rules is essential.
For injuries that are not immediately obvious, like a slow-growing brain tumor, many states apply the “discovery rule.” This means the clock doesn’t start until you discovered your injury and its likely connection to Depo-Provera. Since the link has only recently become widely known through studies published in 2024 and 2025, many women are just now realizing their tumor might be connected to their birth control.
The discovery rule can work in your favor, but it’s not a guarantee. Courts interpret it differently, and some states have “statutes of repose” that set absolute deadlines. The bottom line is not to wait. Speaking with an attorney sooner protects your rights and ensures your claim is filed within the appropriate timeframe.
Time is of the essence with a depo provera injury claim. Taking action now could make all the difference.
Seeking Justice and Compensation
If you’ve developed a meningioma after using Depo-Provera, pursuing a depo provera injury claim is about more than just money—it’s about holding a negligent manufacturer accountable and getting the justice you deserve.
What to Expect in a Depo-Provera Injury Claim
When you file a depo provera injury claim, you’re seeking compensation for the very real harm you’ve suffered. The goal is to make you as whole as possible after a life-altering diagnosis.
Your compensation typically falls into three main categories. Economic damages cover the tangible financial losses you’ve experienced—things like medical bills from diagnosis, surgery, radiation therapy, and ongoing treatment. These can easily run into hundreds of thousands of dollars. Lost wages are also included here, whether you missed work for treatment or had to leave your job entirely due to neurological symptoms that make it impossible to perform your duties.
Non-economic damages address the intangible but equally devastating impacts on your life. This includes compensation for physical pain and suffering—the headaches, seizures, vision loss, and other debilitating symptoms you’ve endured. It also covers emotional distress, the anxiety and depression that often accompany a brain tumor diagnosis, and the loss of quality of life when you can no longer enjoy activities you once loved or maintain the relationships that matter most to you.
In some cases, punitive damages may also be awarded. These aren’t meant to compensate you directly but rather to punish Pfizer for particularly egregious conduct—like knowingly hiding risks from American women while warning patients in other countries. Punitive damages send a strong message that corporate negligence won’t be tolerated.
Every case is unique, and the value of your claim depends on factors like the severity of your tumor, whether you required surgery, your age and earning capacity, and the extent of your ongoing medical needs. To get a better sense of what your case might be worth, check out our Depo-Provera Settlement Calculator and our comprehensive Depo-Provera Lawsuit Compensation Guide 2025.
Pfizer’s Defense Strategy: The Preemption Argument
Pfizer isn’t going to make this easy—they have deep pockets and a team of high-powered lawyers ready to defend against these claims. One of their primary defense strategies is called preemption.
Here’s how the preemption argument works: Pfizer will likely claim that because the FDA approved Depo-Provera and its labeling, they cannot be held liable under state law for failure to warn. Essentially, they’re saying federal regulations “preempt” or override state product liability laws, and since they complied with FDA requirements, they shouldn’t be held responsible.
But this defense has significant weaknesses. First, manufacturers have an ongoing duty to monitor their products and update warnings as new safety information emerges. If Pfizer knew or should have known about the meningioma risk and failed to inform the FDA or push for label changes, preemption doesn’t apply. Second—and this is crucial—Pfizer updated warning labels in Canada and Europe to include meningioma risks but didn’t do the same in the United States. This suggests they had knowledge of the danger and chose not to warn American women adequately.
Plaintiffs’ attorneys are prepared to counter the preemption defense with evidence that Pfizer possessed information about the risks long before they took action, that they failed to conduct adequate post-market surveillance, and that they prioritized profits over patient safety. The discrepancy between international and U.S. warning labels is particularly damning evidence.
For more details on how these legal battles are unfolding, visit our page on What’s the Legal Status of the Depo-Provera Lawsuits?
How Do Legal Fees Work?
One of the biggest concerns people have when considering a lawsuit is: “Can I even afford an attorney?” The good news is that pursuing a depo provera injury claim won’t cost you anything upfront.
The attorneys in our network work on a contingency fee basis. This means you pay nothing unless you win your case or reach a settlement. Your attorney’s fees come directly from your recovery, typically as a percentage (often around 33-40%, depending on whether the case settles or goes to trial). This arrangement levels the playing field, allowing you to take on a pharmaceutical giant like Pfizer without worrying about mounting legal bills.
There are also no upfront costs for things like filing fees, expert witnesses, medical record retrieval, or other case expenses. Your attorney advances these costs and is reimbursed only if your case is successful. This is truly risk-free representation for you.
Additionally, we offer a free case evaluation with no obligation. During this consultation, an experienced attorney will review your situation, explain your legal options, and help you understand whether you have a viable claim—all at no cost to you.
This contingency fee structure exists precisely for cases like these, where individuals who’ve been harmed by large corporations need access to justice without financial barriers. You shouldn’t have to choose between seeking compensation and paying your bills. With our network of attorneys, you don’t have to.
Free Confidential Case Evaluation
Complete the short form below to get an immediate FREE case review with an expert in your specific claim. Don't wait, your case could be time sensitive to file a claim.
Related Posts
Did a North Dakota product cause harm? Understand product liability, your rights, and how to take action for defects.
Get justice for clergy abuse. Find an expert Priest abuse lawyer to navigate complex laws and hold institutions accountable.
Diagnosed with meningioma after Depo-Provera? Understand potential Depo-Provera lawsuit settlements, risks, & how to claim compensation.
Uncover the truth about uber sexual assault cases. Learn about the alarming scale, Uber's accountability, and legal options for justice.
Facing wildfire losses? Discover the best wildfire lawsuit attorneys in California to fight for your full recovery and justice.
Exposed to Roundup & diagnosed with NHL? Discover how to sue Monsanto, understand eligibility, & seek compensation. Your guide to justice.