The Alarming Rise of Birth Control Shot Lawsuits
Birth control shot lawsuit cases are rapidly growing across the United States as hundreds of women come forward with claims that Depo-Provera caused serious brain tumors. These lawsuits allege that Pfizer, the manufacturer, failed to warn patients and doctors about the increased risk of developing meningiomas after using the popular contraceptive injection.
Quick Facts About Birth Control Shot Lawsuits:
- Main Allegation: Depo-Provera increases brain tumor risk by 5.6 times
- Key Evidence: 2024 British Medical Journal study linking prolonged use to meningiomas
- Legal Status: Over 348 cases consolidated in federal court (MDL 3140)
- Who Can File: Women who used Depo-Provera for 1+ years and developed brain tumors
- Potential Compensation: $150,000 to over $1 million depending on injury severity
- Time Limit: 2-3 years from diagnosis in most states
The controversy centers on a failure to warn claim. While Pfizer included meningioma warnings on European labels, U.S. patients received no such warnings despite scientific evidence dating back decades. Women like T.C., who used Depo-Provera for years before developing a meningioma, now live with the constant worry that comes after brain surgery and ongoing neurological issues.
What makes these cases particularly compelling is the stark difference in how Pfizer handled warnings internationally versus domestically. European and Canadian labels mention brain tumor risks, but American women were kept in the dark about these potentially life-threatening side effects.
I’m Mason Arnao, and through my work with Tort Advisor, I’ve helped connect numerous individuals with experienced attorneys for complex legal cases including birth control shot lawsuit claims. My background in technology and lead generation has given me unique insights into how these mass tort cases develop and progress through the legal system.
What is Depo-Provera and What Are the Alleged Health Risks?
For decades, millions of women have relied on Depo-Provera, an injectable contraceptive known as medroxyprogesterone acetate (MPA). It seemed like a perfect solution—effective and convenient. But recent revelations have turned this trusted contraceptive into the center of a growing birth control shot lawsuit storm.
The lawsuits center on two serious brain conditions: meningiomas (brain tumors) and intracranial hypertension (dangerous pressure in the skull). These are life-changing conditions that can require brain surgery and ongoing medical care. To understand the full scope of potential health impacts, you can explore our comprehensive guide on Depo-Provera Severe Side Effects.
How the Birth Control Shot Works
Depo-Provera works by releasing a synthetic hormone called progestin, which prevents pregnancy in two main ways.
First, it prevents ovulation, so no egg is released for fertilization.
Second, it thickens cervical mucus, creating a barrier that is difficult for sperm to penetrate.
Administered every three months, this long-acting contraceptive is a convenient alternative to daily pills, making it popular among many women.
The Link to Brain Tumors: Meningioma
Here’s where things get scary. The current birth control shot lawsuit cases focus on a disturbing findy about Depo-Provera and brain tumors called meningiomas. These tumors grow in the protective membranes surrounding your brain and spinal cord.
A pivotal 2024 study in the British Medical Journal revealed that women using Depo-Provera for over a year had a 5.6 times higher risk of developing meningiomas that require surgery—a significant increase that was not disclosed to patients.
Why does this happen? Scientists believe it’s because many meningiomas have progesterone receptors on the tumors. The synthetic progestin in Depo-Provera may actually feed these tumors, helping them grow larger and more dangerous over time. According to the meningioma information from Mayo Clinic, while these are typically slow-growing tumors, their location in the confined space of your skull means even benign growth can cause serious problems.
The most troubling part? This risk appears strongest with prolonged use – exactly how Depo-Provera is typically prescribed for long-term contraception.
Symptoms You Shouldn’t Ignore
If you’ve used Depo-Provera, especially for more than a year, you need to know the warning signs. These symptoms often start subtle but can become debilitating over time.
Meningioma symptoms include severe headaches that won’t go away, vision changes like blurriness or double vision, and unexpected seizures. Many women also experience memory loss or feel like their thinking isn’t as sharp as it used to be. Hearing loss or tinnitus (ringing in the ears) can develop, along with unexplained weakness in arms or legs.
Intracranial hypertension symptoms present differently but are equally concerning. Women often describe headaches behind the eyes that feel different from regular headaches, persistent ringing in the ears, and frequent dizziness. Nausea that seems to come from nowhere is another red flag.
These symptoms should not be ignored. Treating a meningioma can cost over $700,000, not including the impact on your life and career. If you experience these symptoms after using Depo-Provera, seek immediate medical attention.
Tragically, many women trusted their doctors and Pfizer to warn them of these risks, only to find the dangers after developing life-altering brain conditions.
The Allegations Against Pfizer: A “Failure to Warn”
The birth control shot lawsuit cases boil down to one central issue: did Pfizer do enough to warn women about the serious risks? At the heart of these legal battles is a “failure to warn” claim, and the evidence suggests Pfizer knew about brain tumor risks but kept American women in the dark.
Troublingly, there is a stark difference in how Pfizer handled warnings internationally. European women received clear warnings about brain tumor risks on Depo-Provera labels, while American women did not. This discrepancy raises serious questions about patient safety and the right to informed consent.
What is a “Failure to Warn” Claim?
A failure to warn claim argues that Pfizer had a legal duty to inform patients and doctors about known risks. This includes the manufacturer’s responsibility to test products and monitor safety, the duty to disclose known risks on labels, and the need to provide enough information for informed consent.
Without proper warnings, doctors and patients can’t weigh the benefits against the risks. They might choose a different birth control method or watch for warning signs more carefully. When manufacturers hide or downplay serious risks, they take away that choice.
How Pfizer Allegedly Failed to Inform Patients and Doctors
Scientific evidence suggesting links between progestin hormones and brain tumor growth has existed since the 1980s and 1990s. Yet for decades, Pfizer allegedly chose not to update U.S. drug labels with this crucial information.
The smoking gun? Depo-Provera labels in Europe and Canada already include meningioma warnings. Canadian labels have carried these warnings since at least 2016. European labels mention brain tumor risks too. But American women? They were left completely unaware of these potentially life-threatening side effects.
Plaintiffs allege this was a deliberate choice that prioritized profits over patient safety. Women who trusted Pfizer were unknowingly risking their health because they lacked complete information.
Pfizer often defends itself by claiming the FDA wouldn’t let them add meningioma warnings to U.S. labels. But that doesn’t get them off the hook. If they knew about risks serious enough to warn European women, they had a responsibility to fight harder for American women’s safety too.
A History of Depo-Provera Concerns
This isn’t Depo-Provera’s first rodeo with safety problems. The drug has a troubling history that makes the current brain tumor allegations even more concerning.
In 1978, the FDA reversed Depo-Provera’s approval as a contraceptive due to cancer concerns. It was not re-approved for contraceptive use until 1992.
Then came the 2004 “black box” warning – the FDA’s most serious type of medication warning. This wasn’t for brain tumors, but for significant bone density loss in long-term users. Women using Depo-Provera were at risk for osteoporosis and potentially irreversible bone damage. Again, this was a serious side effect that took years to properly warn patients about.
Pfizer has paid for these oversights before. The company shelled out over $2 million in Canadian settlements for failing to adequately warn about bone density risks. This pattern of inadequate warnings followed by legal consequences is exactly what we’re seeing now with the brain tumor cases.
This history suggests Pfizer knows how to issue proper warnings when required. The current lawsuits question why warnings about brain tumor risks still aren’t reaching American patients.
Understanding the Depo-Provera Birth Control Shot Lawsuit
If you’ve used Depo-Provera and developed a brain tumor, you’re not alone in wondering about your legal options. The current birth control shot lawsuit cases represent a significant opportunity for women who believe they’ve been harmed to seek justice and compensation.
These lawsuits aim to hold Pfizer accountable for its alleged “failure to warn” American women about known brain tumor risks. At Tort Advisor, we understand how overwhelming it can feel to steer these complex legal waters while dealing with a serious health condition. That’s why we connect individuals with experienced attorneys who specialize in pharmaceutical litigation. For comprehensive information about the ongoing legal action, visit our dedicated page on Depo-Provera Lawsuits.
Current Status: The Depo-Provera MDL
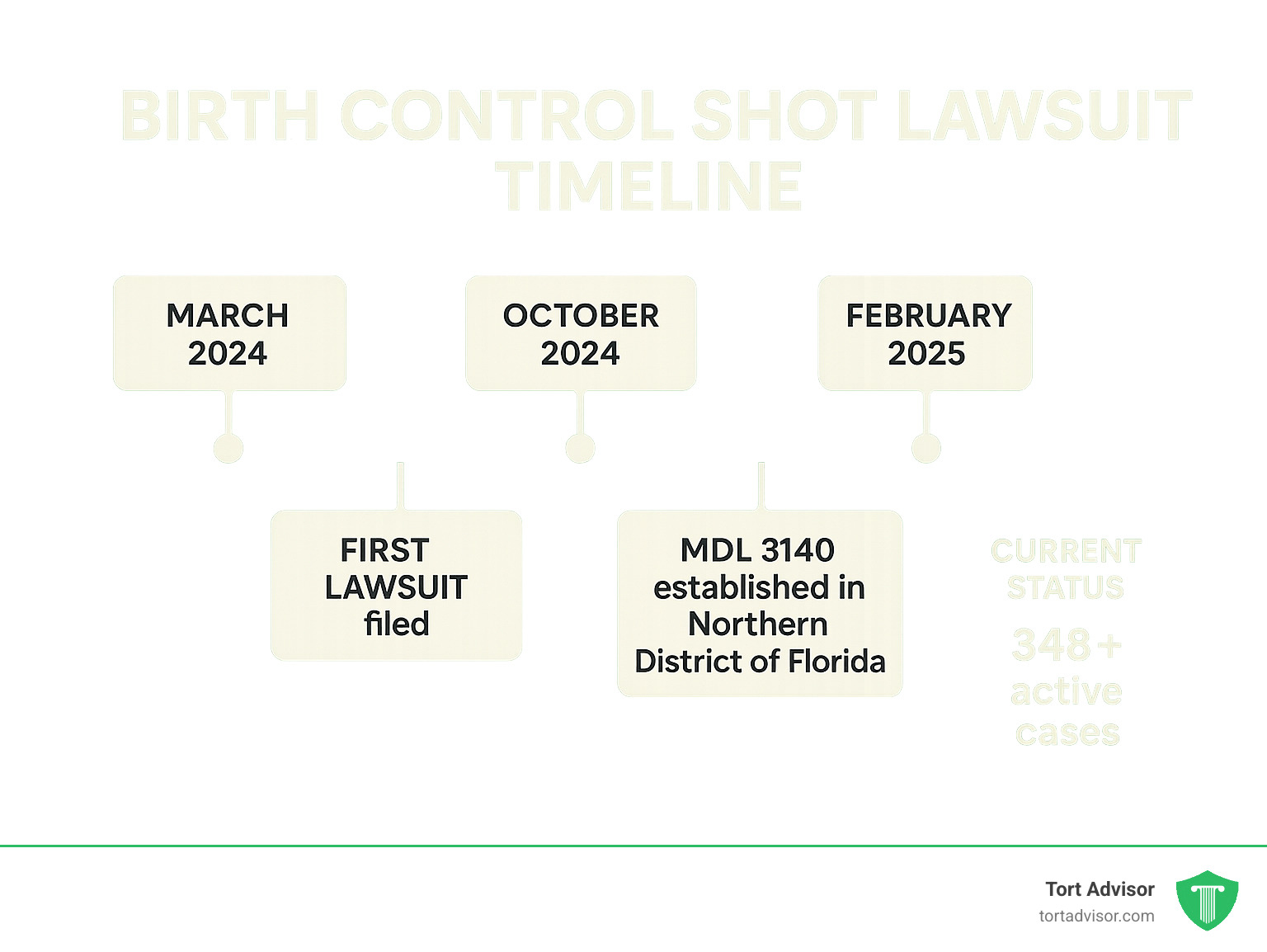
The birth control shot lawsuit cases have reached a crucial milestone with the formation of Multidistrict Litigation (MDL 3140) in the U.S. District Court for the Northern District of Florida. An MDL streamlines hundreds of similar cases that would otherwise clog up courts across the country.
Instead of having scattered cases in different courts, all federal Depo-Provera lawsuits are now consolidated under one experienced judge. Judge M. Casey Rodgers is overseeing the proceedings, and she has a solid track record handling large pharmaceutical cases.
The first Depo-Provera meningioma lawsuit was filed in October 2024. By early 2025, over 348 claims had been filed and consolidated into the MDL, showing significant momentum.
The MDL’s formation is significant, as it signals that the courts recognize common issues in these cases. This streamlines the legal process, leading to more efficient evidence gathering and potentially faster resolutions.
Who Qualifies for a Birth Control Shot Lawsuit?
Not everyone who used Depo-Provera will qualify, but you may have a valid claim if you meet specific criteria.
Duration of use is crucial – most attorneys are looking for women who used Depo-Provera for at least one year. This aligns with research showing that prolonged use significantly increases risk.
A confirmed meningioma diagnosis from medical records is required. This typically involves imaging studies like an MRI and sometimes a biopsy, confirming the tumor’s presence beyond just symptoms.
The severity of your condition matters. Attorneys typically focus on cases where the meningioma required significant medical intervention, such as brain surgery, radiation therapy, or ongoing neurological treatment. Cases involving incidental tumors that don’t require treatment may have limited value.
Timing is everything. Each state has a statute of limitations – typically 2-3 years from when you finded or should have finded that Depo-Provera caused your brain tumor. The “findy rule” may apply, as many women didn’t realize the connection until recently.
Compensation and Potential Settlements in a Birth Control Shot Lawsuit
Potential compensation in a birth control shot lawsuit aims to address the full financial and emotional scope of a brain tumor diagnosis.
Medical expenses often represent the largest component. Brain surgery for meningioma removal can cost upwards of $700,000, and compensation typically covers both past and future medical costs.
Lost wages and earning capacity are also key considerations. Symptoms can derail careers and limit future earning potential. Compensation considers both past and future lost income.
Pain and suffering damages acknowledge the non-economic impacts, such as the fear, anxiety, and effect on your daily life.
While it’s early in the litigation, attorneys predict settlement ranges based on similar pharmaceutical cases. Lower-severity cases might settle for $150,000 to $300,000, while moderate cases could range from $300,000 to $650,000. The most severe cases, involving Grade III meningiomas or permanent neurological damage, could see compensation from $650,000 to over $1.5 million.
These are only estimates. Actual compensation depends on the severity of your tumor, the extent of your treatment, its impact on your life, and the strength of the evidence.
Punitive damages might also be available if a court finds Pfizer’s conduct was particularly reckless, which could be substantial if proven.
Frequently Asked Questions about Depo-Provera Lawsuits
If you’re considering a birth control shot lawsuit, you likely have many questions. Legal matters can be overwhelming, especially when dealing with serious health issues. Here are answers to some of the most common questions.
What is the statute of limitations for filing a Depo-Provera lawsuit?
The statute of limitations is a legal deadline for filing a lawsuit. Missing this deadline can prevent you from filing a claim, regardless of its merit.
These deadlines vary significantly from state to state. Most states give you between 2-3 years from your meningioma diagnosis to file a birth control shot lawsuit. Some states offer more time, while others are stricter.
However, the “findy rule” may apply. Since tumors can develop years after using Depo-Provera, the legal clock may not start until you finded (or should have finded) the link between your meningioma and the shot.
Do not wait to act. Time is critical. Contact an experienced attorney as soon as you suspect a connection to understand your state’s specific deadlines.
How is a Multidistrict Litigation (MDL) different from a class action?
Many people confuse a Multidistrict Litigation (MDL) with a class action lawsuit. The Depo-Provera cases are proceeding as an MDL, which is different in key ways.
An MDL consolidates similar individual lawsuits from across the country before one judge for pretrial proceedings, like evidence gathering. This makes the process more efficient. That’s what is happening with MDL 3140 in Florida.
| Aspect | MDL (What Depo Cases Are) | Class Action |
|---|---|---|
| Your Case | You keep your individual lawsuit with unique circumstances | You become part of one big group lawsuit |
| Settlements | Your compensation is based on your specific injuries and damages | Everyone shares one settlement amount divided among the group |
| Control | You decide whether to accept a settlement or go to trial | Class representatives make decisions for everyone |
| Trial | Your case can go back to your home state for individual trial | The entire group goes to trial as one unit |
The main benefit of an MDL is that your lawsuit remains individual. Your compensation is based on your unique damages, including medical bills and lost wages, not divided among a large group.
Can I sue if I used a generic version of the birth control shot?
Many women used generic versions of medroxyprogesterone acetate. The good news is that you may still have a strong case against Pfizer.
Under a legal concept called “innovator liability,” the original manufacturer (Pfizer) can be held responsible for inadequate warnings, even if you used a generic version, because generic companies must copy the brand-name label.
The legal landscape varies significantly by state. Some states have acceptd innovator liability, while others have not. Additionally, evidence of Pfizer’s involvement in the production and labeling of generic versions could create other avenues for liability.
This is a complex legal issue. An attorney specializing in pharmaceutical law can analyze your state’s laws and determine the best strategy for your birth control shot lawsuit.
What Are Your Next Steps?
If you’ve used Depo-Provera and later developed a meningioma brain tumor or experienced symptoms of intracranial hypertension, you’re probably feeling overwhelmed. Maybe even betrayed. These feelings are completely understandable – you trusted a medication to keep you safe, and now you’re dealing with serious health consequences that you never saw coming.
At Tort Advisor, we get it. We’ve built our entire mission around connecting people like you with the best attorneys who specialize in these exact situations. We don’t work with just anyone – we partner exclusively with highly skilled attorneys who have proven track records in birth control shot lawsuit cases. Because when you’re facing something this serious, you deserve nothing less than the best.
Your health comes first, always. If you haven’t already, make sure you’re working closely with a neurologist or specialist who understands meningiomas. Your medical care is the foundation everything else builds on.
Start gathering your paperwork now. I know it’s not the most exciting task, but collecting your medical records is crucial. You’ll need documentation of your meningioma diagnosis, all your treatment records, and especially your prescription history showing when and how long you used Depo-Provera. Think of these documents as the building blocks of your case – the stronger your documentation, the stronger your position.
Time is not on your side here. This isn’t meant to scare you, but the statute of limitations varies by state, and those deadlines are real. In most states, you have 2-3 years from your diagnosis to file a birth control shot lawsuit, but some states have shorter windows. The findy rule might give you some breathing room, but why take that chance?
We offer completely free case evaluations with no strings attached. No obligation, no pressure – just honest answers about whether you might have a valid claim. Our network spans the entire country, from California to Florida, from Texas to New York, and everywhere in between. Whether you’re in a major city like Chicago or Miami, or somewhere smaller, we can connect you with experienced attorneys right in your area.
The reality is that pharmaceutical companies have teams of lawyers working to protect their interests. You deserve that same level of expertise fighting for yours. These cases are complex, involving medical records, expert witnesses, and intricate legal strategies. It’s not something you want to tackle alone.
Find out if you have a claim by contacting our legal team today. The consultation costs you nothing, but the information you get could be invaluable. Don’t let legal deadlines slip by while you’re trying to figure this out on your own.
You trusted Depo-Provera to be safe. If that trust was misplaced because Pfizer failed to warn you about serious risks, you deserve justice and compensation for what you’ve been through. Let us help you take that first step.
Free Confidential Case Evaluation
Complete the short form below to get an immediate FREE case review with an expert in your specific claim. Don't wait, your case could be time sensitive to file a claim.
Related Posts
Did a North Dakota product cause harm? Understand product liability, your rights, and how to take action for defects.
Get justice for clergy abuse. Find an expert Priest abuse lawyer to navigate complex laws and hold institutions accountable.
Diagnosed with meningioma after Depo-Provera? Understand potential Depo-Provera lawsuit settlements, risks, & how to claim compensation.
Uncover the truth about uber sexual assault cases. Learn about the alarming scale, Uber's accountability, and legal options for justice.
Facing wildfire losses? Discover the best wildfire lawsuit attorneys in California to fight for your full recovery and justice.
Exposed to Roundup & diagnosed with NHL? Discover how to sue Monsanto, understand eligibility, & seek compensation. Your guide to justice.